Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00325
|
|||||
| Drug Name |
Acyclovir
|
|||||
| Synonyms |
2-Amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-6H-purin-6-one; 2-Amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; 2-Amino-9-(2-hydroxy-ethoxymethyl)-1,9-dihydro-purin-6-one; 2-Amino-9-[(2-hydroxyethoxy)methyl]-1,9-dihydro-6H-purin-6-one; 2-amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one; 2-amino-9-{[(2-hydroxyethyl)oxy]methyl}-1,9-dihydro-6H-purin-6-one; 6H-Purin-6-one,2-amino-1,9-dihydro-9-((2-hydroxyethoxy)mehtyl); 9-((2-Hydroxyethoxy)methyl)guanine; 9-(2-Hydroxyethoxy)methylguanine; 9-HYROXYETHOXYMETHYLGUANINE; 9-[(2-Hydroxyethoxy)-methyl]guanine; 9-[(2-Hydroxyethoxy)methyl]guanine; A 4669; ACV; ACV & Pluronic F-68; Aciclovier; Aciclovir (JAN/INN); Aciclovir, Acycloguanosine, Zovirax, Acyclovir; Aciclovirum; Aciclovirum [INN-Latin]; Aciclovirum [Latin]; Acivir (TN); Acivirax (TN); Activir; AcycloFoam; Acycloguanosine; Acyclovir; Acyclovir & Pluronic F-68; Acyclovir (USP); Acyclovir Lauriad; Acyclovir [USAN]; Acyclovir-side chain-2-3H; Alti-Acyclovir; Avirax; B-LF& ACV; BW-248-U; BW-248U; BW-248U74; Bovine lactoferrin & 2-Amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; Cyclovir (TN); DRG-0008; Genvir; Hascovir; Herpex (TN); Maynar; Vipral; Virolex; Viropump; Virorax; W-248-U; Wellcome-248U; Zovir; Zovir (TN); Zovirax; Zovirax (TN); Zovirax Oral Acyclovir (ACV) Suspension
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Herpes simplex virus infection [ICD11:1F00] | Approved | [1] | |||
| Chickenpox [ICD11:1.00E+90] | Approved | [1] | ||||
| Shingles [ICD11:1.00E+91] | Approved | [1] | ||||
| Therapeutic Class |
Antiviral Agents
|
|||||
| Structure |
|
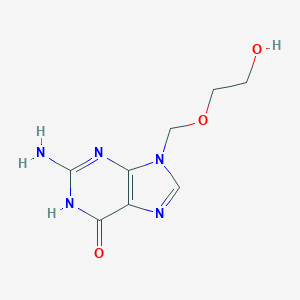 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H11N5O3
|
|||||
| Canonical SMILES |
C1=NC2=C(N1COCCO)N=C(NC2=O)N
|
|||||
| InChI |
InChI=1S/C8H11N5O3/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14/h3,14H,1-2,4H2,(H3,9,11,12,15)
|
|||||
| InChIKey |
MKUXAQIIEYXACX-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 59277-89-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 225.2 | Topological Polar Surface Area | 115 | ||
| Heavy Atom Count | 16 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
-1.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321108
,10589392
,11110742
,11110743
,11335360
,11360599
,11364633
,11367195
,11369757
,11372947
,11374028
,11377919
,11461571
,11466114
,11467234
,11485202
,11485687
,11489379
,11491689
,11492040
,11495553
,11528717
,12013356
,14773677
,15172245
,15220533
,17404601
,1916590
,24278078
,24890562
,25621755
,26612492
,26679648
,26747423
,26747424
,502278
,5344000
,5344001
,594925
,603044
,6855606
,7847289
,7885662
,794369
,7978637
,8150001
,8151390
,829893
,855732
,9029
|
|||||
| ChEBI ID |
ChEBI:2453
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| ASCT2 | Transporter Info | Alanine/serine/cysteine/threonine transporter 2 | Substrate | [3] | ||
| BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [4] | ||
| MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [5] | ||
| MATE2 | Transporter Info | Multidrug and toxin extrusion protein 2 | Substrate | [5] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [6] | ||
| OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | ||
| OAT2 | Transporter Info | Organic anion transporter 2 | Substrate | [7] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [8] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | 1-Oct | Transporter Info | Km =151.2 microM | Proximal tubule (S2) cells-OCT1 | [2] | |
| MATE1 | Transporter Info | Km =2640 microM | Human embryonic kidney cells (HEK293)-MATE1 | [5] | ||
| MATE2 | Transporter Info | Km =4320 microM | Human embryonic kidney cells (HEK293)-MATE2K | [5] | ||
| OAT1 | Transporter Info | Km =839 microM | Human embryonic kidney cells (HEK293)-OAT1 | [8] | ||
| OAT1 | Transporter Info | Km =342 microM | Proximal tubule (S2) cells-OAT1 | [2] | ||
| OAT3 | Transporter Info | Km =772 microM | Human embryonic kidney cells (HEK293)-OCT2 | [8] | ||
| References | ||||||
| 1 | Acyclovir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther. 2002 Mar;300(3):918-24. | |||||
| 3 | Amino Acid Transporter ATB0,+ as a delivery system for drugs and prodrugs. Curr Drug Targets Immune Endocr Metabol Disord. 2005 Dec;5(4):357-64. | |||||
| 4 | Acyclovir is a substrate for the human breast cancer resistance protein (BCRP/ABCG2): implications for renal tubular transport and acyclovir-induced nephrotoxicity. Can J Physiol Pharmacol. 2011 Sep;89(9):675-80. | |||||
| 5 | Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007 Jul 15;74(2):359-71. | |||||
| 6 | Gene expression in the human intestine and correlation with oral valacyclovir pharmacokinetic parameters. J Pharmacol Exp Ther. 2003 Aug;306(2):778-86. | |||||
| 7 | Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol. 2008 Apr;73(4):1151-8. | |||||
| 8 | Expression of organic anion transporter 2 in the human kidney and its potential role in the tubular secretion of guanine-containing antiviral drugs. Drug Metab Dispos. 2012 Mar;40(3):617-24. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
