Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00292
|
|||||
| Drug Name |
Procainamide
|
|||||
| Synonyms |
2-Diethylaminoethylamid kyseliny p-aminobenzoove; 2-Diethylaminoethylamid kyseliny p-aminobenzoove [Czech]; 4-Amino-N-(2-(Diethylamino)Ethyl)Benzamide Sulfate; 4-Amino-N-(2-(diethylamino)ethyl)benzamide; 4-Amino-N-[2-(diethylamino)ethyl]benzamide; 4-amino-N-(2-diethylaminoethyl)-benzamide; 4-amino-N-(2-diethylaminoethyl)benzamide; Benzamide, 4-amino-N-(2-(diethylamino)ethyl)-(9CI); Biocoryl; Novocainamid; Novocainamide; Novocaine amide; Novocamid; P-Amino-N-(2-diethylaminoethyl)benzamide; P-Aminobenzoic diethylaminoethylamide; Procainamida; Procainamida [INN-Spanish]; Procainamide (INN); Procainamide [INN:BAN]; Procainamidum; Procainamidum [INN-Latin]; Procaine amide; Procamide; Procan; Procan (TN); Procanbid; Procanbid (TN); Procapan; Procapan (free base); Pronestyl; Pronestyl (TN); Pronestyl-Sr
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Ventricular arrhythmias [ICD11:BC71] | Approved | [1] | |||
| Therapeutic Class |
Antiarrhythmic Agents
|
|||||
| Structure |
|
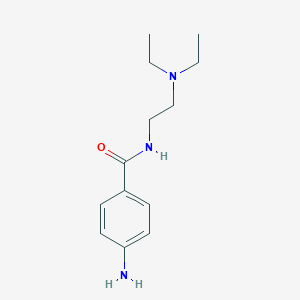 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C13H21N3O
|
|||||
| Canonical SMILES |
CCN(CC)CCNC(=O)C1=CC=C(C=C1)N
|
|||||
| InChI |
InChI=1S/C13H21N3O/c1-3-16(4-2)10-9-15-13(17)11-5-7-12(14)8-6-11/h5-8H,3-4,9-10,14H2,1-2H3,(H,15,17)
|
|||||
| InChIKey |
REQCZEXYDRLIBE-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 51-06-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 235.33 | Topological Polar Surface Area | 58.4 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10524666
,11111690
,11111691
,11120271
,11120759
,11121247
,11121528
,11122008
,11132695
,11336106
,11361345
,11362597
,11363730
,11365159
,11366292
,11367721
,11368854
,11370465
,11370466
,11371829
,11373322
,11374112
,11375883
,11377016
,11382660
,11462317
,11466365
,11467485
,11485065
,11486030
,11489111
,11490371
,11492302
,11494650
,15220788
,26751611
,26751612
,29223991
,3138733
,3296200
,46507313
,47216907
,47440400
,47662439
,47810893
,5227620
,7980387
,8153024
,87927
,9605
|
|||||
| ChEBI ID |
ChEBI:8428
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [3] | ||
| MATE2 | Transporter Info | Multidrug and toxin extrusion protein 2 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MATE1 | Transporter Info | Km =1230 microM | Human embryonic kidney cells (HEK293)-MATE1 | [3] | |
| MATE2 | Transporter Info | Km =1580 microM | Human embryonic kidney cells (HEK293)-MATE2K | [3] | ||
| MATE2 | Transporter Info | Km =4100 microM | Human embryonic kidney cells (HEK293)-MATE2K | [4] | ||
| References | ||||||
| 1 | Procainamide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 3 | Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007 Jul 15;74(2):359-71. | |||||
| 4 | Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006 Aug;17(8):2127-35. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
