Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00044
|
|||||
| Drug Name |
Topotecan
|
|||||
| Synonyms |
(4S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; (S)-10-((Dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione; (S)-10-[(DIMETHYLAMINO)METHYL]-4-ETHYL-4,9-DIHYDROXY-1H-PYRANO[3',4':6,7]INOLIZINO[1,2-B]-QUINOLINE-3,14(4H,12H)-DIONE; (S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]-quinoline-3,14(4H,12H)-dione; (S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; 9-Dimethylaminomethyl-10-hydroxycamptothecin; Hycamptamine; Hycamptin; Hycamtamine; Hycamtin; Hycamtin (TN); SK&F-104864-A; SKF 104864; SKF-104864-A; SKF-S 104864; TOPOTECAN, HYCAMTIN; Topotecan (BAN); Topotecan Monohydrochloride, (S)-Isomer; Topotecan [INN:BAN]; Topotecan lactone; Topotecane; Topotecane [INN-French]; Topotecanum; Topotecanum [INN-Latin]
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Ovarian cancer [ICD11:2C73] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
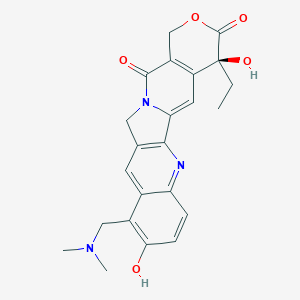 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C23H23N3O5
|
|||||
| Canonical SMILES |
CCC1(C2=C(COC1=O)C(=O)N3CC4=CC5=C(C=CC(=C5CN(C)C)O)N=C4C3=C2)O
|
|||||
| InChI |
InChI=1S/C23H23N3O5/c1-4-23(30)16-8-18-20-12(9-26(18)21(28)15(16)11-31-22(23)29)7-13-14(10-25(2)3)19(27)6-5-17(13)24-20/h5-8,27,30H,4,9-11H2,1-3H3/t23-/m0/s1
|
|||||
| InChIKey |
UCFGDBYHRUNTLO-QHCPKHFHSA-N
|
|||||
| CAS Number |
CAS 119413-54-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 421.4 | Topological Polar Surface Area | 103 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
0.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103170619
,103922004
,103942242
,104321416
,11409161
,11459078
,11461196
,117586312
,124878927
,124878928
,126631363
,126682466
,129347572
,13340
,134337938
,135026276
,135610141
,136342520
,136376449
,137007926
,137275799
,142090317
,144220376
,14807035
,14904851
,152106211
,152198470
,160660968
,160964365
,24769902
,43118056
,46505204
,47348955
,47499101
,47572929
,48242924
,50028360
,50123362
,53790588
,56311903
,57314070
,581639
,606579
,78527173
,7890901
,7980817
,8187013
,92309036
,92710943
,96025302
|
|||||
| ChEBI ID |
CHEBI:63632
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [3] | ||
| MATE2 | Transporter Info | Multidrug and toxin extrusion protein 2 | Substrate | [3] | ||
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [4] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [5] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [6] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | BCRP | Transporter Info | Km =213 microM | Madin-Darby canine kidney cells (MDCKII)-BCRP | [6] | |
| MATE1 | Transporter Info | Km =70 microM | Human embryonic kidney cells (HEK293)-MATE1 | [3] | ||
| MATE2 | Transporter Info | Km =60 microM | Human embryonic kidney cells (HEK293)-MATE2K | [3] | ||
| MRP4 | Transporter Info | Km =1.66 microM | Human liver cancer cells (HepG2)-MRP4 | [5] | ||
| P-GP | Transporter Info | Km =78.3 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [6] | ||
| P-GP | Transporter Info | Km =102 microM | Madin-Darby canine kidney cells (MDCKII)-MDR1 | [6] | ||
| References | ||||||
| 1 | Topotecan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Differential inhibition of murine Bcrp1/Abcg2 and human BCRP/ABCG2 by the mycotoxin fumitremorgin C. Eur J Pharmacol. 2010 Oct 10;644(1-3):41-8. | |||||
| 3 | Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007 Jul 15;74(2):359-71. | |||||
| 4 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
| 5 | Topotecan is a substrate for multidrug resistance associated protein 4. Curr Drug Metab. 2006 Jan;7(1):105-18. | |||||
| 6 | Involvement of P-glycoprotein, multidrug resistance protein 2 and breast cancer resistance protein in the transport of belotecan and topotecan in Caco-2 and MDCKII cells. Pharm Res. 2008 Nov;25(11):2601-12. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
