Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00037
|
|||||
| Drug Name |
Cimetidine
|
|||||
| Synonyms |
1-Cyano-2-methyl-3-(2-(((5-methyl-4-imidazolyl)methyl)thio)ethyl)guanidine; 1-Cyano-2-methyl-3-[2-[[(5-methylimidazol-4-yl)methyl]thio]ethyl]guanidine; 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine; 2-Cyano-1-methyl-3-(2-(((5-methylimidazol-4-yl)methyl)thio)ethyl)guanidine; 2-Cyano-1-methyl-3-[2-(5-methyl-1H-imidazol-4-yl-methylthio)ethyl]guanidine; 2-cyano-1-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine; 2-cyano-1-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; Acibilin; Acinil; Altramet; Biomet400; Brumetidina; C 4522; CIMETIDINE A/AB; Ci metum; Cimal; Cimetadine; Cimetag; Cimetidina; Cimetidina [INN-Spanish]; Cimetidine (JP15/USP/INN); Cimetidine Hcl; Cimetidine [USAN:INN:BAN:JAN]; Cimetidinum; Cimetidinum [INN-Latin]; Cimetum; DRG-0150; Dyspamet; Edalene; Eureceptor; Evicer; FPF 1002; Gastrobitan; Gastromet; Histodil; Magicul; Metracin; N''-Cyano-N-methyl-N'-[2-[(5-methyl-1H-imidazol-4-yl)methylthio]ethyl]guanidine; N''-cyano-N-methyl-N'-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)-ethyl)guanidine; N''-cyano-N-methyl-N'-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; N-Cyano-N'-Methyl-N''-(2-(((5-Methyl-1H-Imidazol-4-YL)Methyl)Thio)Ethyl) Guanidine; N-Cyano-N'-methyl-N''-(2-(((5-methyl-1 H-imidazol-4-yl) methyl)thio)ethyl)guanidine; N-Cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)guanidine; N-Cyano-N'-methyl-[2-[[[5-methyl-1H-imidazol-4-yl]methyl]thio]ethyl]guanidine; N-cyano-N'-methyl-N''-(2-([(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl)ethyl)guanidine; N-cyano-N'-methyl-N''-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; Peptol; SK&F-92334; SKF 92334; SKF-92334; Sigmetadine; Tagamet; Tagamet (TN); Tagamet HB (TN); Tagamet HB200 (TN); Tagamet Hb; Tagamet Hb 200; Tagamet, SKF-92334, Tratul, Tametin, Dyspamet, Acinil, Cimetidine; Tametin; Tratul; Ulcedin; Ulcedine; Ulcestop; Ulcimet; Ulcofalk; Ulcomedina; Ulcomet; Ulhys; Valmagen; Venopex
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acid reflux disorder [ICD11:DA22] | Approved | [1] | |||
| Therapeutic Class |
Antiulcer Agents
|
|||||
| Structure |
|
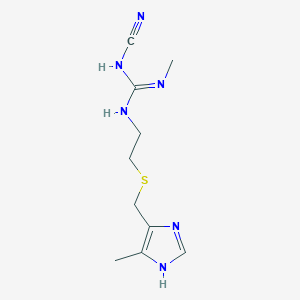 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C10H16N6S
|
|||||
| Canonical SMILES |
CC1=C(N=CN1)CSCCNC(=NC)NC#N
|
|||||
| InChI |
InChI=1S/C10H16N6S/c1-8-9(16-7-15-8)5-17-4-3-13-10(12-2)14-6-11/h7H,3-5H2,1-2H3,(H,15,16)(H2,12,13,14)
|
|||||
| InChIKey |
AQIXAKUUQRKLND-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 51481-61-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 252.34 | Topological Polar Surface Area | 114 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
0.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321166
,10524846
,11110948
,11113773
,11335341
,11360580
,11363389
,11365951
,11368513
,11372297
,11374462
,11376675
,11407328
,11461552
,11484620
,11488598
,11491204
,11492627
,11494309
,11533056
,12013445
,14774387
,15122273
,15196790
,17389962
,17404856
,22391437
,24277766
,24531048
,26612025
,26679798
,26747359
,26747360
,26751956
,26751957
,29221911
,4266417
,4493559
,460110
,46487919
,46505360
,47275096
,615111
,6699439
,6897918
,7847361
,7978946
,8147032
,8149633
,9167
|
|||||
| ChEBI ID |
ChEBI:3699
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| 2-Oct | Transporter Info | Organic cation transporter 2 | Substrate | [3] | ||
| BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [4] | ||
| MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [5] | ||
| MATE2 | Transporter Info | Multidrug and toxin extrusion protein 2 | Substrate | [5] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [7] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | 2-Oct | Transporter Info | Km =60 microM | Human embryonic kidney cells (HEK293)-OCT2 | [2] | |
| 2-Oct | Transporter Info | Km =72.6 microM | Human embryonic kidney cells (HEK293)-OCT2 | [8] | ||
| MATE1 | Transporter Info | Km =170 microM | Human embryonic kidney cells (HEK293)-MATE1 | [5] | ||
| MATE2 | Transporter Info | Km =120 microM | Human embryonic kidney cells (HEK293)-MATE2K | [5] | ||
| MATE2 | Transporter Info | Km =370 microM | Human embryonic kidney cells (HEK293)-MATE2K | [9] | ||
| OAT3 | Transporter Info | Km =174 microM | Chinese hamster ovary (CHO) cells-OAT3 | [6] | ||
| OAT3 | Transporter Info | Km =113 microM | Human embryonic kidney cells (HEK293)-OAT3 | [10] | ||
| OAT3 | Transporter Info | Km =149 microM | Human embryonic kidney cells (HEK293)-OAT3 | [11] | ||
| OAT3 | Transporter Info | Km =57.4 microM | Oocytes-OAT3 | [12] | ||
| References | ||||||
| 1 | Cimetidine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 3 | Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: the role of renal organic cation transporter OCT2. Drug Metab Pharmacokinet. 2010;25(4):328-34. | |||||
| 4 | Progesterone acts via progesterone receptors A and B to regulate breast cancer resistance protein expression. Mol Pharmacol. 2008 Mar;73(3):613-5. | |||||
| 5 | Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007 Jul 15;74(2):359-71. | |||||
| 6 | Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther. 2007 May;321(2):673-83. | |||||
| 7 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 8 | A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther. 2005 Oct;315(1):337-45. | |||||
| 9 | Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006 Aug;17(8):2127-35. | |||||
| 10 | Inhibition of oat3-mediated renal uptake as a mechanism for drug-drug interaction between fexofenadine and probenecid. Drug Metab Dispos. 2006 May;34(5):743-7. | |||||
| 11 | Is the monkey an appropriate animal model to examine drug-drug interactions involving renal clearance? Effect of probenecid on the renal elimination of H2 receptor antagonists. J Pharmacol Exp Ther. 2006 Mar;316(3):1187-94. | |||||
| 12 | Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001 May;59(5):1277-86. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
