Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00030
|
|||||
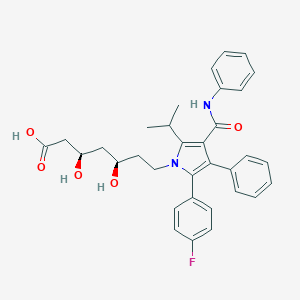
| Drug Name |
Atorvastatin
|
|||||
| Synonyms |
(3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (3R,5R)-7-[3-(anilinocarbonyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (R-(R*,R*))-2-(4-Fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-1H-pyrrole-1-heptanoic acid; (betaR,deltaR)-2-(p-Fluorophenyl)-beta,delta-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoic acid; 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL]-3,5-DIHYDROXY-HEPTANOIC ACID; Atogal; Atorlip; Atorpic; Atorvastatin (INN); Atorvastatin [INN:BAN]; Atorvastatina; Atorvastatine; Atorvastatinium; Atrovastin; Cardyl; Faboxim; Lipitor (TN); Lipitor(TM); Lipotropic; Lipovastatinklonal; Liprimar; Lowden; Sincol; Sortis; Sortis (TN); Sotis; Torvacard; Torvast; Totalip; Tozalip; Tulip; Vastina; Xanator; Xarator; Xavator; Zurinel
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Heart attack [ICD11:BA41.Z] | Approved | [1] | |||
| Dyslipidaemias [ICD11:5C8Z] | Approved | [1] | ||||
| Hyperlipidaemia [ICD11:5C8Z] | Approved | [1] | ||||
| Therapeutic Class |
Anticholesteremic Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C33H35FN2O5
|
|||||
| Canonical SMILES |
CC(C)C1=C(C(=C(N1CCC(CC(CC(=O)O)O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4
|
|||||
| InChI |
InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m1/s1
|
|||||
| InChIKey |
XUKUURHRXDUEBC-KAYWLYCHSA-N
|
|||||
| CAS Number |
CAS 134523-00-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 558.6 | Topological Polar Surface Area | 112 | ||
| Heavy Atom Count | 41 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103554720
,104178840
,104321734
,104829550
,117367061
,124892211
,126525305
,126624171
,126658151
,126682126
,127315782
,127315783
,127315784
,127315785
,127315786
,127315787
,127315788
,127315789
,127315790
,127315791
,127315792
,127315793
,14910832
,26684326
,26697359
,29215408
,43118161
,49845979
,50037926
,51091801
,53787715
,56311281
,56311722
,56311943
,56311969
,56312802
,56313042
,56313334
,56313578
,56314463
,57314133
,75432172
,7884979
,7978735
,8187078
,822166
,85856289
,9052
,92714388
,93166494
|
|||||
| ChEBI ID |
ChEBI:39548
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | ABCG1 | Transporter Info | ATP-binding cassette sub-family G member 1 | Substrate | [2] | |
| MCT1 | Transporter Info | Monocarboxylate transporter 1 | Substrate | [3] | ||
| MCT2 | Transporter Info | Monocarboxylate transporter 2 | Substrate | [4] | ||
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [5] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [6] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [7] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [8] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [9] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1B1 | Transporter Info | Km =0.77 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [10] | |
| OATP1B1 | Transporter Info | Km =12.4 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [11] | ||
| OATP1B1 | Transporter Info | Km =18.9 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [12] | ||
| OATP1B3 | Transporter Info | Km =0.73 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [10] | ||
| OATP2B1 | Transporter Info | Km =2.84 microM | Human embryonic kidney cells (HEK293)-OATP2B1 | [10] | ||
| OATP2B1 | Transporter Info | Km =0.2 microM | Madin-Darby canine kidney cells (MDCKII)-OATP2B1 | [13] | ||
| References | ||||||
| 1 | Ramipril was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | HMG-CoA reductase inhibitors, simvastatin and atorvastatin, downregulate ABCG1-mediated cholesterol efflux in human macrophages. J Cardiovasc Pharmacol. 2013 Jul;62(1):90-8. | |||||
| 3 | H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol. 2007 Jul;92(4):603-19. | |||||
| 4 | Monocarboxylate Transporters in Drug Disposition: Role in the Toxicokinetics and Toxicodynamics of the Drug of Abuse GHB. | |||||
| 5 | Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol. 2010 Dec 1;80(11):1746-53. | |||||
| 6 | Rifampicin alters atorvastatin plasma concentration on the basis of SLCO1B1 521T>C polymorphism. Clin Chim Acta. 2009 Jul;405(1-2):49-52. | |||||
| 7 | FDA Drug Development and Drug Interactions | |||||
| 8 | Human platelets express organic anion-transporting peptide 2B1, an uptake transporter for atorvastatin. Drug Metab Dispos. 2009 May;37(5):1129-37. | |||||
| 9 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
| 10 | Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem. 2012 May 24;55(10):4740-63. | |||||
| 11 | Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005 Jul;15(7):513-22. | |||||
| 12 | effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007 Feb;81(2):194-204. | |||||
| 13 | Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006 Dec;80(6):607-20. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
