Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00027
|
|||||
| Drug Name |
Ganciclovir
|
|||||
| Synonyms |
2'-NDG; 2'-Nor-2'-deoxyguanosine; 2-(6-Amino-purin-9-ylmethoxy)-propane-1,3-diol; 2-Amino-1,9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)-6-H-purin-6-one; 2-Amino-9-(2-hydroxy-1-hydroxymethyl-ethoxymethyl)-1,9-dihydro-purin-6-one; 2-amino-9-((1,3-dihydroxypropan-2-yloxy)methyl)-1H-purin-6(9H)-one; 2-amino-9-((1,3-dihydroxypropan-2-yloxy)methyl)-3H-purin-6(9H)-one; 2-amino-9-((1,3-dihydroxypropan-2-yloxy)methyl)-9H-purin-6-ol; 2-amino-9-(1,3-dihydroxypropan-2-yloxymethyl)-3H-purin-6-one; 2-amino-9-(2-hydroxy-1-hydroxymethylethoxymethyl)-6,9-dihydro-1H-6-purinone; 2-amino-9-{[(1,3-dihydroxypropan-2-yl)oxy]methyl}-1,9-dihydro-6H-purin-6-one; 2-amino-9-{[(1,3-dihydroxypropan-2-yl)oxy]methyl}-6,9-dihydro-3H-purin-6-one; 9-((1,3-Dihydroxy-2-propoxy)methyl)guanine; 9-((2-Hydroxy-1-(hydroxymethyl)ethoxy)methyl)guanine; 9-(1,3-DIHYDROXY-PROPOXYMETHANE)GUANINE; 9-[(1,3-Dihydroxy-2-propoxy)methyl]guanine; BIOLF-62; BW 759; BW 759U; BW-759U; BW-795; BW-B 759U; Biolf 62; Citovirax; Cymevan; Cymeven; Cymevene; Cymevene (TN); Cytovene; Cytovene (TN); Cytovene-IV; DRG-0018; G 2536; GA2; GANCICLOVIR SODIUM; GCV; GCV & 1110U81; GCV & MSL; Ganciclovir & C34-dgA immunotoxin; Ganciclovir & D5-dgA immunotoxin; Ganciclovir (JAN/USP/INN); Ganciclovir [USAN:INN:BAN:JAN]; Ganciclovirum; Ganciclovirum [Latin]; Gancyclovir; Guanine, 9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)-and MSL, neutralizing monoclonal antibody; HHEMG; Hydroxyacyclovir; IN1478; MB3795; RS-21592; ST-605; Virgan; Vitrasert; Vitrasert (TN); Zirgan
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Cytomegalovirus infections [ICD11:1D82] | Approved | [1] | |||
| Therapeutic Class |
Antiviral Agents
|
|||||
| Structure |
|
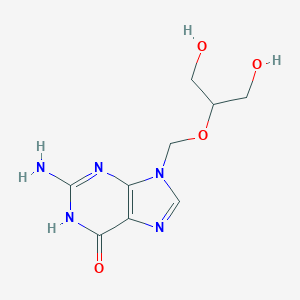 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C9H13N5O4
|
|||||
| Canonical SMILES |
C1=NC2=C(N1COC(CO)CO)N=C(NC2=O)N
|
|||||
| InChI |
InChI=1S/C9H13N5O4/c10-9-12-7-6(8(17)13-9)11-3-14(7)4-18-5(1-15)2-16/h3,5,15-16H,1-2,4H2,(H3,10,12,13,17)
|
|||||
| InChIKey |
IRSCQMHQWWYFCW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 82410-32-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 255.23 | Topological Polar Surface Area | 135 | ||
| Heavy Atom Count | 18 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
-2.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321977
,11111224
,11111225
,11466867
,11467987
,11486454
,12013652
,14749970
,14798783
,16217245
,17405102
,23929519
,24278453
,25622077
,26758755
,29222588
,3720872
,46507294
,47216865
,47515408
,47589082
,48185084
,48185085
,48334596
,48404464
,48404465
,49681203
,49698657
,49974979
,5006228
,50106328
,50106329
,50113251
,53777645
,53800918
,56312143
,56312588
,56424026
,57321811
,617697
,7298558
,76491727
,7840003
,7847399
,7887702
,7979369
,81041223
,8139883
,85231063
,866342
|
|||||
| ChEBI ID |
ChEBI:465284
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| ASCT2 | Transporter Info | Alanine/serine/cysteine/threonine transporter 2 | Substrate | [3] | ||
| BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [4] | ||
| MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [5] | ||
| MATE2 | Transporter Info | Multidrug and toxin extrusion protein 2 | Substrate | [5] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [6] | ||
| MRP5 | Transporter Info | Multidrug resistance-associated protein 5 | Substrate | [7] | ||
| OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [8] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | 1-Oct | Transporter Info | Km =516.2 microM | Proximal tubule (S2) cells-OCT1 | [2] | |
| MATE1 | Transporter Info | Km =5120 microM | Human embryonic kidney cells (HEK293)-MATE1 | [5] | ||
| MATE2 | Transporter Info | Km =4280 microM | Human embryonic kidney cells (HEK293)-MATE2K | [5] | ||
| OAT1 | Transporter Info | Km =896 microM | Proximal tubule (S2) cells-OAT1 | [2] | ||
| References | ||||||
| 1 | Valganciclovir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther. 2002 Mar;300(3):918-24. | |||||
| 3 | Amino Acid Transporter ATB0,+ as a delivery system for drugs and prodrugs. Curr Drug Targets Immune Endocr Metabol Disord. 2005 Dec;5(4):357-64. | |||||
| 4 | Side populations of glioblastoma cells are less sensitive to HSV-TK/GCV suicide gene therapy system than the non-side population. In Vitro Cell Dev Biol Anim. 2010 Jun;46(6):497-501. | |||||
| 5 | Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007 Jul 15;74(2):359-71. | |||||
| 6 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
| 7 | Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab Rev. 2005;37(1):253-78. | |||||
| 8 | FDA Drug Development and Drug Interactions | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
