Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01489
|
|||||
| Drug Name |
Hydroxycamptothecin
|
|||||
| Synonyms |
10-Hydroxycamptothecin; 19685-09-7; (S)-10-Hydroxycamptothecin; Hydroxycamptothecin; 10-hydroxycamptothecine; 10-Hydroxy camptothecin; Hydroxycamptothecine; Camptothecin, hydroxy-; 10-Hydroxy-Camptothecin; (S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; Camptothecin, 10-hydroxy-; Camptothecine, 10-hydroxy-; UNII-9Z01632KRV; NSC 107124; HCPT; (4S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; (S)-10-Hydroxycamptothecin hydrate; NSC107124
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Solid tumours [ICD11:2D4Z] | Phase 1 | [1] | |||
| Structure |
|
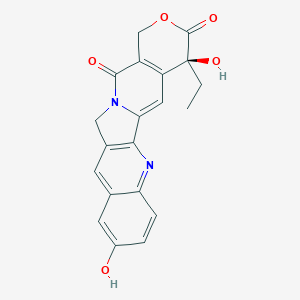 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C20H16N2O5
|
|||||
| Canonical SMILES |
CCC1(C2=C(COC1=O)C(=O)N3CC4=C(C3=C2)N=C5C=CC(=CC5=C4)O)O
|
|||||
| InChI |
InChI=1S/C20H16N2O5/c1-2-20(26)14-7-16-17-11(5-10-6-12(23)3-4-15(10)21-17)8-22(16)18(24)13(14)9-27-19(20)25/h3-7,23,26H,2,8-9H2,1H3/t20-/m0/s1
|
|||||
| InChIKey |
HAWSQZCWOQZXHI-FQEVSTJZSA-N
|
|||||
| CAS Number |
CAS 19685-09-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 364.4 | Topological Polar Surface Area | 100 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
0.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
407052
,645392
,8140019
,10228777
,11342034
,11362217
,11364533
,11367095
,11369657
,11372600
,11375589
,11377819
,11404568
,11485555
,11487619
,11489476
,11491436
,11493629
,11495453
,12015125
,12173672
,16124509
,26612627
,26680421
,26750003
,26758893
,44427566
,47348452
,48392929
,49831587
,50028362
,50107786
,50122774
,53787862
,53790463
,57336414
,80013607
,81093235
,85788508
,89360248
,92721280
,96024160
,103071286
,103082955
,103170545
,103922002
,104420324
,117586316
,124636619
,124891994
|
|||||
| ChEBI ID |
CHEBI:81395
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT01202370) A Phase I Study of AR-67 (7-t-butyldimethylsilyl-10-hydroxycamptothecin) Given on Days 1, 4 8, 12 & 15 of an Every 21-day Cycle in Adult Patients With Refractory or Metastatic Solid Malignancies | |||||
| 2 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
