Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01419
|
|||||
| Drug Name |
Methysergide
|
|||||
| Synonyms |
Deseril; Desernil; Desernyl; Deseryl; Desril; Dimethylergometrin; Methylmethylergonovine; Methysergid; Methysergidum; Metisergide; Metisergido; Sansert; Methyllysergic acid butanolamide; Metisergide [DCIT]; UML 491; Deseril (TN); Methysergidum [INN-Latin]; Metisergido [INN-Spanish]; Sansert (TN); UML-491; Methysergide (USAN/INN); Methysergide [USAN:INN:BAN]; N-(alpha-(Hydroxymethyl)propyl)-1-methyl-dextro-lysergamide; N-(1-(Hydroxymethyl)propyl)-1-methyl-dextro-(+)-lysergamide; (+)-9,10-Didehydro-N-(1-(hydroxymethyl)propyl)-1,6-dimethylergoline-8beta-carboxamide; (+)-N-(1-(Hydroxymethyl)propyl)-1-methyl-D-lysergamide; (8beta)-N-[(1S)-1-(hydroxymethyl)propyl]-1,6-dimethyl-9,10-didehydroergoline-8-carboxamide; 1-Methyl-D-lysergic acid butanolamide; 1-Methyl-dextro-lysergic acid (+)-1-hydroxy-2-butylamide; 1-Methylmethylergonovine; 9,10-Didehydro-N-(1-(hydroxymethyl)propyl)-1,6-dimethylergoline-8-carboxamide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Migraine [ICD11:8A80] | Approved | [1] | |||
| Therapeutic Class |
Vasoconstrictor Agents
|
|||||
| Structure |
|
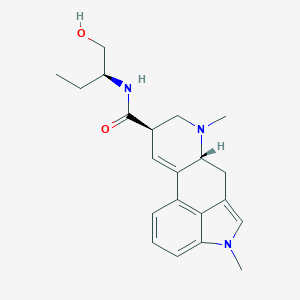 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H27N3O2
|
|||||
| Canonical SMILES |
CCC(CO)NC(=O)C1CN(C2CC3=CN(C4=CC=CC(=C34)C2=C1)C)C
|
|||||
| InChI |
InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1
|
|||||
| InChIKey |
KPJZHOPZRAFDTN-ZRGWGRIASA-N
|
|||||
| CAS Number |
CAS 361-37-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 353.5 | Topological Polar Surface Area | 57.5 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
2.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
9408
,7849416
,7979958
,8156955
,26751590
,29228253
,47365379
,47515489
,48334682
,48416266
,49965410
,50104228
,50428725
,53790023
,57325757
,85787459
,90340563
,92309300
,103292415
,103940495
,104321196
,124750057
,124886858
,124886859
,128415730
,134337676
,134973635
,135650596
,137002477
,144204446
,160963595
,170464684
,175266387
,176484551
,179236187
,221673418
,226426779
,252614966
|
|||||
| ChEBI ID |
CHEBI:92629
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Methysergide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther. 2002 Dec;303(3):1029-37. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
