Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01377
|
|||||
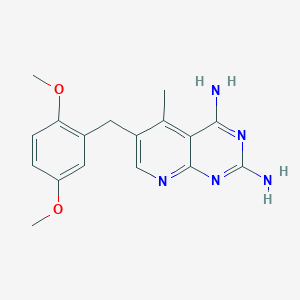
| Drug Name |
Piritrexim
|
|||||
| Synonyms |
Piritrexim; 72732-56-0; Piritrexim [INN]; Piritreximum [Latin]; Piritrexime [French]; 6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine-2,4-diamine; Piritrexima [Spanish]; BW 301U; UNII-MK2A783ZUT; BW-301U; TCMDC-137235; BRN 5768301; MK2A783ZUT; CHEMBL7492; 2,4-Diamino-5-methyl-6-(2,5-dimethoxybenzyl)pyrido(2,3-d)pyrimidine; 6-(2,5-DIMETHOXY-BENZYL)-5-METHYL-PYRIDO[2,3-D]PYRIMIDINE-2,4-DIAMINE; 6-((2,5-Dimethoxyphenyl)methyl)-5-methylpyrido(2,3-d)pyrimidine-2,4-diamine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Urethral cancer [ICD11:2F78] | Phase 2 | [1] | |||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H19N5O2
|
|||||
| Canonical SMILES |
CC1=C2C(=NC(=NC2=NC=C1CC3=C(C=CC(=C3)OC)OC)N)N
|
|||||
| InChI |
InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22)
|
|||||
| InChIKey |
VJXSSYDSOJBUAV-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 72732-56-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 325.4 | Topological Polar Surface Area | 109 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
2.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
585461
,596153
,5321568
,7889090
,8183612
,12012913
,14801638
,26704486
,34718371
,46506046
,46518330
,50035075
,50293384
,85866344
,93579418
,103165437
,103855306
,104304731
,126420216
,129485784
,134340716
,135029179
,137008239
,143157948
,160966790
,163724435
,172896495
,178103986
,179151032
,198977797
,208012006
,219402188
,223518945
,225249588
,226399387
,241132020
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT00002914) Piritrexim in Treating Patients With Advanced Cancer of the Urinary Tract | |||||
| 2 | Mutant Gly482 and Thr482 ABCG2 mediate high-level resistance to lipophilic antifolates. Cancer Chemother Pharmacol. 2006 Dec;58(6):826-34. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
