Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01365
|
|||||
| Drug Name |
ABT-263
|
|||||
| Synonyms |
Navitoclax; ABT 263; S1001_Selleck; ABT263, Navitoclax; 4-(4-{[2-(4-chlorophenyl)-5,5-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({4-({(1R)-3-morpholin-4-yl-1-[(phenylsulfanyl)methyl]propyl}amino)-3-[(trifluoromethyl)sulfonyl]phenyl}sulfonyl)benzamide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Myelofibrosis [ICD11:2A20.2] | Phase 2 | [1] | |||
| Structure |
|
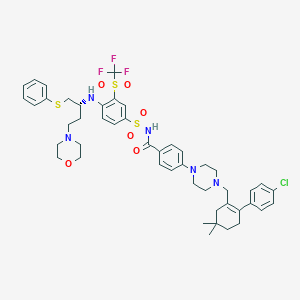 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C47H55ClF3N5O6S3
|
|||||
| Canonical SMILES |
CC1(CCC(=C(C1)CN2CCN(CC2)C3=CC=C(C=C3)C(=O)NS(=O)(=O)C4=CC(=C(C=C4)NC(CCN5CCOCC5)CSC6=CC=CC=C6)S(=O)(=O)C(F)(F)F)C7=CC=C(C=C7)Cl)C
|
|||||
| InChI |
InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1
|
|||||
| InChIKey |
JLYAXFNOILIKPP-KXQOOQHDSA-N
|
|||||
| CAS Number |
CAS 119229-65-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 974.6 | Topological Polar Surface Area | 170 | ||
| Heavy Atom Count | 65 | Rotatable Bond Count | 16 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 14 | |||
| XLogP |
9.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
56311703
,56314453
,57304426
,57504587
,87219189
,87457108
,96099448
,99003698
,99245529
,99246089
,99431767
,99460835
,103640740
,104115920
,124756924
,124899202
,124899203
,125163731
,126583646
,126667000
,126724167
,131465096
,131465716
,134221945
,135263748
,135626669
,135727392
,136920253
,137126833
,141663047
,143499177
,152035716
,152164573
,152240012
,152258081
,152344157
,160646920
,162011689
,162037376
,162202714
,163123180
,163821637
,164194132
,164831796
,174007035
,174531509
,198955115
,204380851
,223388517
,223685382
|
|||||
| ChEBI ID |
CHEBI:94128
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT03222609) A Study Evaluating Tolerability and Efficacy of Navitoclax in Combination With Ruxolitinib in Subjects With Myelofibrosis | |||||
| 2 | The B-cell lymphoma 2 (BCL2)-inhibitors, ABT-737 and ABT-263, are substrates for P-glycoprotein. Biochem Biophys Res Commun. 2011 May 6;408(2):344-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
