Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01277
|
|||||
| Drug Name |
Entecavir
|
|||||
| Synonyms |
2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one; 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-1,9-dihydro-6H-purin-6-one; 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-1,9-dihydro-6H-purin-6-one-water (1/1); 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3H-purin-6-one; 6-H-Purin-6-one-,2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]; 9-((1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl)guanine monohydrate; BMS-200475; Baraclude; Baraclude (TN); ETV; Entecavir (INN); Entecavir (USAN); Entecavir hydrate; Entecavir hydrate (JAN); Entecavir monohydrate; SQ-34676
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic hepatitis B infection [ICD11:1E51.0] | Approved | [1] | |||
| Therapeutic Class |
Antiviral Agents
|
|||||
| Structure |
|
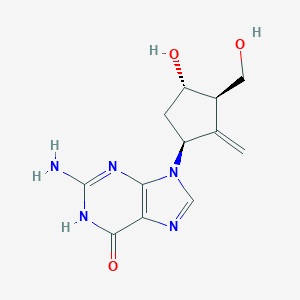 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C12H15N5O3
|
|||||
| Canonical SMILES |
C=C1C(CC(C1CO)O)N2C=NC3=C2N=C(NC3=O)N
|
|||||
| InChI |
InChI=1S/C12H15N5O3/c1-5-6(3-18)8(19)2-7(5)17-4-14-9-10(17)15-12(13)16-11(9)20/h4,6-8,18-19H,1-3H2,(H3,13,15,16,20)/t6-,7-,8-/m0/s1
|
|||||
| InChIKey |
QDGZDCVAUDNJFG-FXQIFTODSA-N
|
|||||
| CAS Number |
CAS 142217-69-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 277.28 | Topological Polar Surface Area | 126 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
-1.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
17398002
,37101953
,49830936
,56391942
,57379683
,91749140
,99319526
,104253461
,118313779
,124757591
,125001900
,125164395
,126651626
,134222146
,136920321
,137129051
,137179356
,152034465
,162011500
,162183301
,163370951
,163908003
,164825059
,164833113
,172087090
,172440023
,174531851
,175265673
,180672743
,186013131
,196106268
,198991663
,210274904
,210280539
,223379249
,223398838
,226415922
,249739265
,251893489
,252110220
,252162333
,252316076
|
|||||
| ChEBI ID |
CHEBI:473990
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 3-Oct | Transporter Info | Organic cation transporter 3 | Substrate | [2] | |
| BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | ||
| CNT2 | Transporter Info | Concentrative nucleoside transporter 2 | Substrate | [2] | ||
| CNT3 | Transporter Info | Concentrative Na(+)-nucleoside cotransporter 3 | Substrate | [2] | ||
| ENT1 | Transporter Info | Equilibrative nucleoside transporter 1 | Substrate | [2] | ||
| ENT2 | Transporter Info | Equilibrative nucleoside transporter 2 | Substrate | [2] | ||
| OAT2 | Transporter Info | Organic anion transporter 2 | Substrate | [3] | ||
| OCTN2 | Transporter Info | Organic cation/carnitine transporter 2 | Substrate | [2] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | CNT2 | Transporter Info | Km =53.2 microM | Madin-Darby canine kidney (MDCK) cells-hCNT2 | [2] | |
| CNT3 | Transporter Info | Km =23.1 microM | Madin-Darby canine kidney (MDCK) cells-hCNT3 | [2] | ||
| References | ||||||
| 1 | Entecavir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Multiple SLC and ABC Transporters Contribute to the Placental Transfer of Entecavir. Drug Metab Dispos. 2017 Mar;45(3):269-278. | |||||
| 3 | Human organic anion transporter 2 is an entecavir, but not tenofovir, transporter. Drug Metab Pharmacokinet. 2017 Feb;32(1):116-119. | |||||
| 4 | The oligopeptide transporter 2-mediated reabsorption of entecavir in rat kidney. Eur J Pharm Sci. 2014 Feb 14;52:41-7. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
