Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01208
|
|||||
| Drug Name |
Eprosartan
|
|||||
| Synonyms |
(4-carboxybenzyl)imidazole-5-acrylic acid; (E)-2-Butyl-1-(p-carboxybenzyl)-alpha-2-thenylimidazole-5-acrylic acid; (E)-3-[2-n-butyl-1-{(4-carboxyphenyl)methyl}-1H-imidazol-5-yl]-2-(2-thienyl)methyl-2-propenoic acid; (E)-4-((2-Butyl-5-(2-carboxy-3-(thiophen-2-yl)prop-1-en-1-yl)-1H-imidazol-1-yl)methyl)benzoic acid; (E)-alpha{[2-butyl-1-[(4-carboxyphenyl)methyl]-1H-imidazole-5-yl]methylene}-2-thiopheneproprionic acid; 133040-01-4; 2KH13Z0S0Y; 4-({2-butyl-5-[(1E)-2-carboxy-3-(2-thienyl)prop-1-en-1-yl]-1H-imidazol-1-yl}methyl)benzoic acid; 4-({2-butyl-5-[(1E)-2-carboxy-3-(thiophen-2-yl)prop-1-en-1-yl]-1H-imidazol-1-yl}methyl)benzoic acid; 4-[[2-butyl-5-[(E)-3-hydroxy-3-oxo-2-(thiophen-2-ylmethyl)prop-1-enyl]imidazol-1-yl]methyl]benzoic acid; CHEBI:4814; CHEMBL813; Eprosartan (USAN/INN); Eprosartan [USAN:BAN:INN]; F 108566; F-108566; SK&F 108566; SK&F-108566; SK-108566; SKF-108566; Teveten; Teveten (TN); UNII-2KH13Z0S0Y; [3H]eprosartan
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11:BA00] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
| Structure |
|
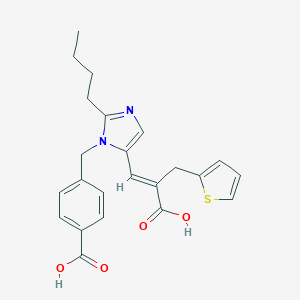 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C23H24N2O4S
|
|||||
| Canonical SMILES |
CCCCC1=NC=C(N1CC2=CC=C(C=C2)C(=O)O)C=C(CC3=CC=CS3)C(=O)O
|
|||||
| InChI |
InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+
|
|||||
| InChIKey |
OROAFUQRIXKEMV-LDADJPATSA-N
|
|||||
| CAS Number |
CAS 133040-01-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 424.5 | Topological Polar Surface Area | 121 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
4.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103213560
,103924435
,113854438
,117814116
,121277912
,126682167
,127329712
,127329713
,127329714
,127329715
,127329716
,127329717
,127329718
,127329719
,127329720
,127329721
,127329722
,127329723
,131835100
,134223577
,134338185
,135026481
,135650236
,136367951
,137002491
,142300349
,144115951
,14929623
,162161427
,163389404
,164814942
,17398013
,175266444
,176484078
,26757976
,39289969
,46506765
,48415948
,49658621
,49830795
,50064350
,50303490
,7979177
,81092802
,85209073
,8616481
,91612944
,92308966
,93166188
,9670
|
|||||
| ChEBI ID |
ChEBI:4814
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [2] | |
| References | ||||||
| 1 | Eprosartan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | High-affinity interaction of sartans with H+/peptide transporters. Drug Metab Dispos. 2009 Jan;37(1):143-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
