Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01203
|
|||||
| Drug Name |
Dactinomycin
|
|||||
| Synonyms |
1H-Pyrrolo(2,1-1)-(1,4,7,10,13)oxatetraazacyclohexadecine; 4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-dicarboxamide; ACT D; ACTINOMYCIN D AMP; AD (VAN); Actactinomycin A IV; Actinomycin 11 cosmegen; Actinomycin 7; Actinomycin A IV; Actinomycin Aiv; Actinomycin C (sub1); Actinomycin C(sub1); Actinomycin C1; Actinomycin D (JP15); Actinomycin D deriv. of 3H-phenoxaocardazine; Actinomycin D, sodium deoxyribonucleic acid complex; Actinomycin I; Actinomycin I (sub1); Actinomycin I(sub 1); Actinomycin I(sub1); Actinomycin I1; Actinomycin IV; Actinomycin X 1; Actinomycin X1; Actinomycin cl; Actinomycin x i; Actinomycin-(threo-val-pro-sar-meval); Actinomycin-IV; Actinomycin-[threo-val-pro-sar-meval]; Actinomycindioic D acid, dilactone; Actinomyein-theo-val-pro-sar-meval; Acto-D; Antibiotic from Streptomyces parvullus; COSMEGEN (TN); Chounghwamycin B; Cosmegen; D Actinomycin; DVA-DPR-SAR-MVA-(c1)DTH-PXZ-(c11)DTH-DVA-DPR-SAR-MVA; Dactinomicina; Dactinomicina [INN-Spanish]; Dactinomycin (USP); Dactinomycin D; Dactinomycin [USAN:BAN]; Dactinomycine; Dactinomycine [INN-French]; Dactinomycinum; Dactinomycinum [INN-Latin]; Dactinomyein d; Dilactone actin omycindioic D acid; Dilactone actinomycin D acid; Dilactone actinomycindioic D acid; GNF-PF-1977; HBF 386; HBF 386 meractinomycin; Lyovac cosmegen; Meractinomycin; NP-005932; O)-(1-oxo-1,2-ethanediyl)]bis(N-methyl)L-valine; Oncostatin K; PXZ-THR-DVA-PRO-SAR-MVA-THR-DVA-PRO-SAR-MVA; X 97
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Cancer [ICD11:2A00-2F9Z] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
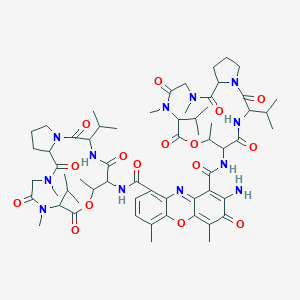 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C62H86N12O16
|
|||||
| Canonical SMILES |
CC1C(C(=O)NC(C(=O)N2CCCC2C(=O)N(CC(=O)N(C(C(=O)O1)C(C)C)C)C)C(C)C)NC(=O)C3=C4C(=C(C=C3)C)OC5=C(C(=O)C(=C(C5=N4)C(=O)NC6C(OC(=O)C(N(C(=O)CN(C(=O)C7CCCN7C(=O)C(NC6=O)C(C)C)C)C)C(C)C)C)N)C
|
|||||
| InChI |
InChI=1S/C62H86N12O16/c1-27(2)42-59(84)73-23-17-19-36(73)57(82)69(13)25-38(75)71(15)48(29(5)6)61(86)88-33(11)44(55(80)65-42)67-53(78)35-22-21-31(9)51-46(35)64-47-40(41(63)50(77)32(10)52(47)90-51)54(79)68-45-34(12)89-62(87)49(30(7)8)72(16)39(76)26-70(14)58(83)37-20-18-24-74(37)60(85)43(28(3)4)66-56(45)81/h21-22,27-30,33-34,36-37,42-45,48-49H,17-20,23-26,63H2,1-16H3,(H,65,80)(H,66,81)(H,67,78)(H,68,79)
|
|||||
| InChIKey |
RJURFGZVJUQBHK-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 50-76-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 1255.4 | Topological Polar Surface Area | 356 | ||
| Heavy Atom Count | 90 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 18 | |||
| XLogP |
3.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103185659
,103934097
,104299382
,11120223
,11120711
,11121199
,11147306
,11434891
,124766124
,124886988
,125537037
,125767589
,126687205
,131332481
,134337828
,137156344
,137545216
,160964308
,161005131
,162022425
,162022855
,164787451
,174007348
,26752119
,29221206
,3141480
,47216557
,47515096
,47588774
,47959487
,48034857
,48034858
,49854365
,50114308
,51071907
,520465
,5343980
,565029
,57321102
,57392818
,74382088
,75117937
,7847281
,8136899
,8151387
,841047
,85083371
,85789491
,8990
,95152991
|
|||||
| ChEBI ID |
ChEBI:27666
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| References | ||||||
| 1 | Actinomycin D was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Mol Pharmacol. 2003 Jan;63(1):65-72. | |||||
| 3 | Expression of multidrug resistance-associated protein in NIH/3T3 cells confers multidrug resistance associated with increased drug efflux and altered intracellular drug distribution. Cancer Res. 1995 Nov 15;55(22):5342-7. | |||||
| 4 | Potential role of drug transporters in the pathogenesis of medically intractable epilepsy. Epilepsia. 2005 Feb;46(2):224-35. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
