Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00708
|
|||||
| Drug Name |
Roxithromycin
|
|||||
| Synonyms |
9-(O-((2-Methoxyethoxy)methyl)oxime)erythromycin; 9-[O-(2-methoxyethoxymethyl)-oxime] of erythromycin; Assoral; Biaxsig (TN); Coroxin (TN); Erythromycin 9-(-O-[2-methoxyethoxy]methyloxime);Erythromycin 9-(O-((2-methoxyethoxy)methyl)oxime); Erythromycin, 9-(O-((2-methoxyethoxy)methyl)oxime); Overall; RC2952; RU 28965; RU 965; RU-28965; RU-965; RXM; Rossitrol; Roxar (TN); Roximycin (TN); Roxithromycin & Tumor Necrosis Factor; Roxithromycin (JP15/USAN/INN); Roxithromycin [USAN:INN:JAN]; Roxithromycine; Roxithromycine [French]; Roxithromycinum; Roxithromycinum [Latin]; Roxitromicina; Roxitromicina [Spanish]; Roxl-150 (TN); Roxo (TN); Rulid; Rulide; Rulide (TN); Surlid; Surlid (TN); Tirabicin (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Bacterial infections [ICD11:1A00-1H0Z] | Withdrawn | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
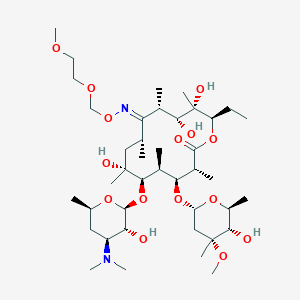
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C41H76N2O15
|
|||||
| Canonical SMILES |
CCC1C(C(C(C(=NOCOCCOC)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)O)C)C)O)(C)O
|
|||||
| InChI |
InChI=1S/C41H76N2O15/c1-15-29-41(10,49)34(45)24(4)31(42-53-21-52-17-16-50-13)22(2)19-39(8,48)36(58-38-32(44)28(43(11)12)18-23(3)54-38)25(5)33(26(6)37(47)56-29)57-30-20-40(9,51-14)35(46)27(7)55-30/h22-30,32-36,38,44-46,48-49H,15-21H2,1-14H3/b42-31+/t22-,23-,24+,25+,26-,27+,28+,29-,30+,32-,33+,34-,35+,36-,38+,39-,40-,41-/m1/s1
|
|||||
| InChIKey |
RXZBMPWDPOLZGW-XMRMVWPWSA-N
|
|||||
| CAS Number |
CAS 80214-83-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 837 | Topological Polar Surface Area | 217 | ||
| Heavy Atom Count | 58 | Rotatable Bond Count | 13 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 17 | |||
| XLogP |
3.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
104240364
,114784612
,124637170
,135013062
,137003693
,137241413
,144075595
,14791576
,14816129
,152109149
,162107641
,17193218
,176251435
,179038902
,179113863
,210279582
,210281905
,226447059
,252401199
,26612461
,26681158
,43527885
,46507676
,47500552
,48020029
,48020030
,49658614
,49658887
,49835235
,50123162
,50599965
,53787485
,56314318
,57288831
,57371618
,585237
,602768
,7848773
,7890303
,81093210
,8149971
,88283688
,92124863
,92307881
,99301470
|
|||||
| ChEBI ID |
ChEBI:48935
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018 Jan 4;46(D1):D1091-D1106. (familyId=1465) | |||||
| 2 | Differences in assessment of macrolide interaction with human MDR1 (ABCB1, P-gp) using rhodamine-123 efflux, ATPase activity and cellular accumulation assays. Eur J Pharm Sci. 2010 Sep 11;41(1):86-95. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
