Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00705
|
|||||
| Drug Name |
Astemizole
|
|||||
| Synonyms |
1-(p-Fluorobenzyl)-2-((1-(p-methoxyphenethyl)-4-piperidyl)amino)benzimidazole; AlacanBrand of Astemizole; Alermizol; Alonga Brand of Astemizole; Alonga, Astemizol; Astemina; Astemisan; Astemisol; Astemison; Astemizol; Astemizol Alonga; Astemizol [German]; Astemizol [INN-Spanish]; Astemizol ratiopharm; Astemizole (JAN/USP/INN); Astemizole Alacan Brand; Astemizole Alonga Brand; Astemizole Byk Brand; Astemizole Diba Brand; Astemizole Elfar Brand; Astemizole Esteve Brand; Astemizole Fustery Brand; Astemizole ICN Brand; Astemizole Janssen Brand; Astemizole Lesvi Brand; Astemizole McNeil Brand; Astemizole Medinsa Brand; Astemizole Merck Brand; Astemizole Senosiain Brand; Astemizole Septa Brand; Astemizole Smaller Brand; Astemizole Urbion Brand; Astemizole Vita Brand; Astemizole [USAN:BAN:INN]; Astemizole ratiopharm Brand; Astemizolum; Astemizolum [INN-Latin]; Astesen; Byk Brand of Astemizole; Diba Brand of Astemizole; Elfar Brand of Astemizole; Emdar; Esmacen;Fustermizol; Esteve Brand of Astemizole; Fustery Brand of Astemizole; GNF-PF-2461; HISMANAL (TN); Hestazol; Hestazol, Kelp, Laridal, Retolen, Wareezol, HSBD 6799, BRN 4830190; Hismanal; Histamen; Histaminos; Histazol; Hubermizol; ICN Brand of Astemizole; Janssen Brand of Astemizole; Kelp; Laridal; Lesvi Brand of Astemizole; MJD-30; McNeil Brand of Astemizole; Medinsa Brand of Astemizole; Merck Brand of Astemizole; Metodih; Metodik; Nono-Nastizol A; Novo-mastizol A; Paralergin; R 42512; R 43 512; R-43-512; R-43512; R43512; Ratiopharm Brand of Astemizole; Ratiopharm, Astemizol; Reig Jofre Brand of Astemizole; Retolen; Rifedot; Rimbol; Romadin; Senosiain Brand of Astemizole; Septa Brand of Astemizole; Simprox; Smaller Brand of Astemizole; Urbion Brand of Astemizole; Urdrim; Vita Brand of Astemizole; Wareezol; Waruzol; [3H]Astemizole
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Allergic rhinitis [ICD11:CA08.0] | Withdrawn | [1] | |||
| Therapeutic Class |
Antiallergic Agents
|
|||||
| Structure |
|
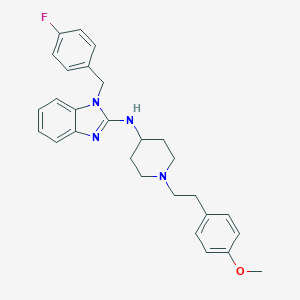 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C28H31FN4O
|
|||||
| Canonical SMILES |
COC1=CC=C(C=C1)CCN2CCC(CC2)NC3=NC4=CC=CC=C4N3CC5=CC=C(C=C5)F
|
|||||
| InChI |
InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31)
|
|||||
| InChIKey |
GXDALQBWZGODGZ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 68844-77-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 458.6 | Topological Polar Surface Area | 42.3 | ||
| Heavy Atom Count | 34 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321136
,11112828
,11335214
,11360453
,11363342
,11365904
,11368466
,11372003
,11374824
,11376628
,11461425
,11466164
,11467284
,11485540
,11485887
,11489548
,11490887
,11493037
,11494262
,11528621
,11538010
,12013338
,14760289
,22391432
,26613163
,26680817
,26746979
,26746980
,26751496
,26751497
,29221421
,4266413
,459037
,46487928
,46508569
,47216611
,47440072
,47515146
,47736292
,47810587
,47885241
,48034934
,48259050
,5313663
,7847301
,7978732
,8150150
,8151518
,855746
,9050
|
|||||
| ChEBI ID |
CHEBI:2896
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Comparative tolerability of second generation antihistamines. Drug Saf. 1999 May;20(5):385-401. | |||||
| 2 | Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
