Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00693
|
|||||
| Drug Name |
Prazosin
|
|||||
| Synonyms |
1-(3-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanyl-carbonyl)piperazine hydrochloride; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl) piperazine; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine; 2-(4-(2-Furoyl)piperazin-1-yl)-4-amino-6,7-dimethoxyquinazoline; 2-[4-(2-furoyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine; 4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazinyl 2-furyl ketone; CP-12299; Furazosin; Hypovase (TN); Justac; Lentopres; Minipress (TN); Piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)-(8CI); Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)-(9CI); Prazocin; Prazosin (INN); Prazosin HCl; Prazosin [INN:BAN]; Prazosina; Prazosina [INN-Spanish]; Prazosine; Prazosine [INN-French]; Prazosinum; Prazosinum [INN-Latin]; TNP00312; Vasoflex (TN); [3H]-Prazosin; [4-(4-amino-6,7-dimethoxy-quinazolin-2-yl)piperazin-1-yl]-(2-furyl)methanone; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](furan-2-yl)methanone; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(furan-2-yl)methanone
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11:BA00] | Approved | [1] | |||
| Severe congestive heart failure [ICD11:BD10] | Approved | [1] | ||||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
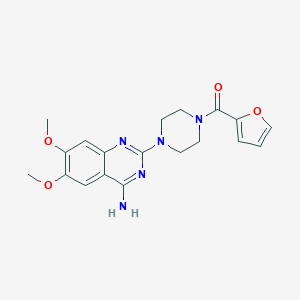
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H21N5O4
|
|||||
| Canonical SMILES |
COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCN(CC3)C(=O)C4=CC=CO4)N)OC
|
|||||
| InChI |
InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22)
|
|||||
| InChIKey |
IENZQIKPVFGBNW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 19216-56-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 383.4 | Topological Polar Surface Area | 107 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11112649
,11112650
,11113367
,11120245
,11120733
,11121221
,11121703
,11122183
,11335550
,11360789
,11362790
,11363716
,11365352
,11366278
,11367914
,11368840
,11370831
,11370832
,11371821
,11373515
,11374104
,11376076
,11377002
,11406810
,11461761
,11466975
,11468095
,11485033
,11486809
,11489105
,11490367
,11492298
,11494636
,14804776
,26751613
,26751614
,29223971
,4500854
,46508594
,47364941
,47364942
,47588782
,47662032
,47662033
,47662034
,47662035
,7980376
,8153011
,841974
,9572
|
|||||
| ChEBI ID |
ChEBI:8364
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km =20 microM | High five cells-MDR1 | [4] | |
| References | ||||||
| 1 | Prazosin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006 Mar 8;234(1):4-33. | |||||
| 3 | P-glycoprotein and breast cancer resistance protein expression and function at the blood-brain barrier and blood-cerebrospinal fluid barrier (choroid plexus) in streptozotocin-induced diabetes in rats. Brain Res. 2011 Jan 25;1370:238-45. | |||||
| 4 | Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10594-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
