Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00643
|
|||||
| Drug Name |
Lansoprazole
|
|||||
| Synonyms |
lansoprazole; Prevacid; Monolitum; Bamalite; Lansoprazol; Limpidex; Agopton; Ogastro; Opiren; Lanzor; Prevacid SoluTab; Takepron; Lanzopral; Zoton; Lansoprazolum; Lasoprol; Mesactol; Lansopep; Lanproton; Lancid; Ketian; Prezal; Lanston; Aprazol; Blason; Ulpax; Lanz; Pro Ulco; AG-1749; Compraz; Suprecid; Prosogan; Ilsatec; Promp; Zoprol; Dakar; Prevacid Iv; Ogast; Lanzol-30; AG 1749; Lansoprazol [INN-Spanish]; Lansoprazolum [INN-Latin]; Lansox; Lanzo; A-65006; Prevacid NapraPAC; Prevacid 24HR; Lansoprazole [USAN:BAN:INN]; HSDB 7204
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Peptic ulcer [ICD11:DA61] | Approved | [1] | |||
| Therapeutic Class |
Antiulcer Agents
|
|||||
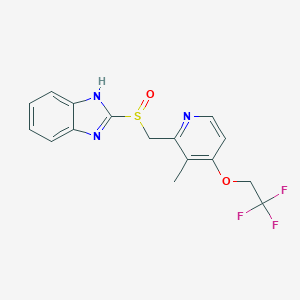
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H14F3N3O2S
|
|||||
| Canonical SMILES |
CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3N2)OCC(F)(F)F
|
|||||
| InChI |
InChI=1S/C16H14F3N3O2S/c1-10-13(20-7-6-14(10)24-9-16(17,18)19)8-25(23)15-21-11-4-2-3-5-12(11)22-15/h2-7H,8-9H2,1H3,(H,21,22)
|
|||||
| InChIKey |
MJIHNNLFOKEZEW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 103577-45-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 369.4 | Topological Polar Surface Area | 87.1 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
2.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
104179070
,104234371
,126682042
,128604168
,135156589
,137931058
,140767605
,14901842
,15375021
,162189572
,164178109
,164761527
,170503333
,175265917
,175269426
,175611039
,178102134
,184527042
,185965235
,210274918
,210280553
,223658647
,223798807
,226429139
,23998121
,241033947
,251879797
,252151427
,252431524
,252811271
,25819922
,44715913
,80665304
,89736101
,96025586
|
|||||
| ChEBI ID |
ChEBI:6375
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Lansoprazole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | A novel screening strategy to identify ABCB1 substrates and inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2009 Jan;379(1):11-26. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
