Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00638
|
|||||
| Drug Name |
Grepafloxacin
|
|||||
| Synonyms |
(+-)-1-Cyclopropyl-6-fluoro-1,4-dihydro-5-methyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-Cyclopropyl-6-fluoro-1,4-dihydro-5-methyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-cyclopropyl-6-fluoro-5-methyl-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid; Grepafloxacin (unspecified); Grepafloxacin [INN]; Raxar; Raxar (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic bronchitis [ICD11:CA20.1] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
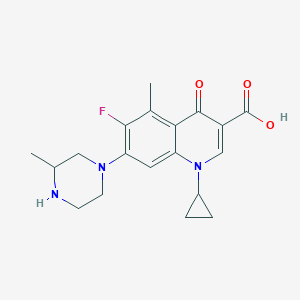 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H22FN3O3
|
|||||
| Canonical SMILES |
CC1CN(CCN1)C2=C(C(=C3C(=C2)N(C=C(C3=O)C(=O)O)C4CC4)C)F
|
|||||
| InChI |
InChI=1S/C19H22FN3O3/c1-10-8-22(6-5-21-10)15-7-14-16(11(2)17(15)20)18(24)13(19(25)26)9-23(14)12-3-4-12/h7,9-10,12,21H,3-6,8H2,1-2H3,(H,25,26)
|
|||||
| InChIKey |
AIJTTZAVMXIJGM-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 119914-60-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 359.4 | Topological Polar Surface Area | 72.9 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-0.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103179577
,104011329
,104353024
,124766026
,125536480
,127411146
,134338274
,135029665
,13542
,137044453
,143019201
,14852426
,160963712
,162793470
,163693542
,163852853
,179148061
,184545887
,184546334
,223439875
,224916848
,226420671
,241036011
,242059760
,43128629
,46507253
,50042505
,50112780
,50471882
,53788063
,57318735
,603312
,6610845
,8195206
|
|||||
| ChEBI ID |
CHEBI:5543
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [5] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [6] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km =580 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [7] | |
| References | ||||||
| 1 | Grepafloxacin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones. Drug Metab Dispos. 2007 Oct;35(10):1873-9. | |||||
| 3 | Limited distribution of new quinolone antibacterial agents into brain caused by multiple efflux transporters at the blood-brain barrier. J Pharmacol Exp Ther. 2000 Oct;295(1):146-52. | |||||
| 4 | Fluoroquinolone efflux mediated by ABC transporters. J Pharm Sci. 2008 Sep;97(9):3483-93. | |||||
| 5 | Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007 Jan-Feb;4(1):85-94. | |||||
| 6 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 7 | Secretory mechanisms of grepafloxacin and levofloxacin in the human intestinal cell line caco-2. J Pharmacol Exp Ther. 2000 Oct;295(1):360-6. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
