Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00454
|
|||||
| Drug Name |
Sunitinib
|
|||||
| Synonyms |
5-(5-FLUORO-2-OXO-1,2-DIHYDRO-INDOL-3-YLIDENEMETHYL)-2,4-DIMETHYL-1H-PYRROLE-3-CARBOXYLIC ACID (2-DIETHYLAMINO-ETHYL)-AMIDE; KS-5022; N-(2-diethylaminoethyl)-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide; N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide; PDGF TK antagonist; SU-11248J; SU-12662; SU11248; Su-011248; Sunitanib; Sunitinib (INN); Sunitinib (Pan-TK inhibitor); Sunitinib (free base); Sunitinibum; Sutent (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Advanced renal cell carcinoma [ICD11:2C90] | Approved | [1] | |||
| Imatinib-resistant gastrointestinal stromal tumor [ICD11:2B5B] | Approved | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
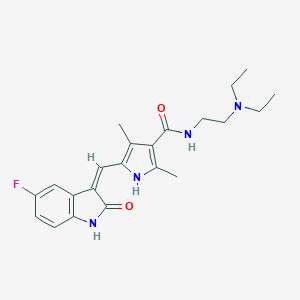 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H27FN4O2
|
|||||
| Canonical SMILES |
CCN(CC)CCNC(=O)C1=C(NC(=C1C)C=C2C3=C(C=CC(=C3)F)NC2=O)C
|
|||||
| InChI |
InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12-
|
|||||
| InChIKey |
WINHZLLDWRZWRT-ATVHPVEESA-N
|
|||||
| CAS Number |
CAS 557795-19-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 398.5 | Topological Polar Surface Area | 77.2 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
2.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103175813
,104178853
,104223163
,11061329
,113911638
,118049563
,118855335
,124893175
,124893176
,126663102
,134222706
,134337335
,134339174
,135236614
,136367772
,137001819
,137255559
,137255561
,140170598
,14720359
,14830382
,152047612
,160964598
,162012027
,162184461
,163371066
,163395369
,164045165
,164825281
,164825282
,172085035
,172091423
,172919594
,176484660
,176485072
,26675759
,26758053
,39301727
,46507140
,50070562
,50071184
,50100120
,57361294
,8034462
,80856906
,85171053
,91148451
,93581003
,96025237
,99443888
|
|||||
| ChEBI ID |
ChEBI:38940
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| RALBP1 | Transporter Info | RalBP1-associated Eps domain-containing protein 2 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | RALBP1 | Transporter Info | Km =7.2 microM | Human kidney cancer cells (Caki-2)-RALBP1 | [5] | |
| References | ||||||
| 1 | Sunitinib malate was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Double-transduced MDCKII cells to study human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) interplay in drug transport across the blood-brain barrier. Mol Pharm. 2011 Apr 4;8(2):571-82. | |||||
| 3 | Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013 Mar 15;19(6):1458-66. | |||||
| 4 | Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin Cancer Res. 2009 Oct 1;15(19):6062-9. | |||||
| 5 | Rlip76 transports sunitinib and sorafenib and mediates drug resistance in kidney cancer. Int J Cancer. 2010 Mar 15;126(6):1327-38. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
