Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00432
|
|||||
| Drug Name |
Temsirolimus
|
|||||
| Synonyms |
Torisel
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Renal cell carcinoma [ICD11:2C90] | Approved | [1] | |||
| Structure |
|
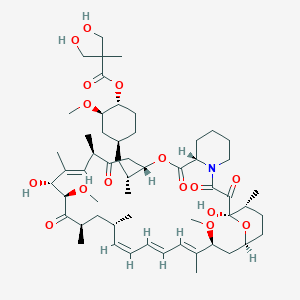 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C56H87NO16
|
|||||
| Canonical SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OC(=O)C(C)(CO)CO)C)C)O)OC)C)C)C)OC
|
|||||
| InChI |
InChI=1S/C56H87NO16/c1-33-17-13-12-14-18-34(2)45(68-9)29-41-22-20-39(7)56(67,73-41)51(63)52(64)57-24-16-15-19-42(57)53(65)71-46(30-43(60)35(3)26-38(6)49(62)50(70-11)48(61)37(5)25-33)36(4)27-40-21-23-44(47(28-40)69-10)72-54(66)55(8,31-58)32-59/h12-14,17-18,26,33,35-37,39-42,44-47,49-50,58-59,62,67H,15-16,19-25,27-32H2,1-11H3/b14-12+,17-13+,34-18+,38-26+/t33-,35-,36-,37-,39-,40+,41+,42+,44-,45+,46+,47-,49-,50+,56-/m1/s1
|
|||||
| InChIKey |
CBPNZQVSJQDFBE-FUXHJELOSA-N
|
|||||
| CAS Number |
CAS 162635-04-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 1030.3 | Topological Polar Surface Area | 242 | ||
| Heavy Atom Count | 73 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 16 | |||
| XLogP |
5.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103771165
,12014940
,124756959
,125163765
,131346349
,135727446
,136345868
,137005644
,139209825
,139209826
,142578760
,144206068
,14816760
,14914596
,160967858
,162108260
,162172159
,174527830
,175437917
,176484822
,198991980
,204359550
,226408727
,249896045
,252158731
,50112771
,56313948
,91616133
,99436955
|
|||||
| ChEBI ID |
CHEBI:79699
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Temsirolimus was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Association of NR1I2, CYP3A5 and ABCB1 genetic polymorphisms with variability of temsirolimus pharmacokinetics and toxicity in patients with metastatic bladder cancer. Cancer Chemother Pharmacol. 2017 Sep;80(3):653-659. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
