Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00429
|
|||||
| Drug Name |
Losartan
|
|||||
| Synonyms |
(2-butyl-4-chloro-1-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-1H-imidazol-5-yl)methanol; (2-butyl-4-chloro-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-imidazol-5-yl)methanol; 1H-Imidazole-5-methanol, 2-butyl-4-chloro-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-(9CI); 2-Butyl-4-chloro-1-((2'-(1H-etrazol-5-yl) (1,1'-biphenyl)-4-yl)methyl)-1H-imidazole-5-methanol; 2-Butyl-4-chloro-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl-1H-imidazole-5-methanol; 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5ylphenyl)benzyl]imidazole-5-methanol; 2-n-butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]imidazole; 2-n-butyl-4-chloro-5-hydroxymethyl-1-[[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl]methyl]imidazole; CL23623; Cozaar; Cozaar (TN); DUP 89; DuP-753; Hyzaar; LOSARTAN POTASSIUM; Lortaan; Losartan (INN); Losartan [INN:BAN]; Losartan monopotassium salt; Losartic; Losartic (TN); MK-954; MK954; [2-butyl-5-chloro-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11:BA00] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
| Structure |
|
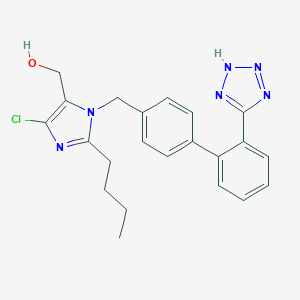 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H23ClN6O
|
|||||
| Canonical SMILES |
CCCCC1=NC(=C(N1CC2=CC=C(C=C2)C3=CC=CC=C3C4=NNN=N4)CO)Cl
|
|||||
| InChI |
InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28)
|
|||||
| InChIKey |
PSIFNNKUMBGKDQ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 114798-26-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 422.9 | Topological Polar Surface Area | 92.5 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
4.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103546496
,104086517
,104305027
,11364607
,11367169
,11369731
,11372014
,11374749
,11377893
,11485629
,11489493
,11490813
,11492940
,11495527
,118212888
,14758375
,14880416
,26612696
,26680690
,26748956
,26748957
,29223075
,46506538
,46530544
,47773674
,48072880
,48221855
,48416186
,4964191
,49979780
,50107496
,50150720
,53789286
,56312013
,56314181
,57322070
,6595486
,7979808
,8152487
,842088
,85209145
,85789659
,91011664
,92124757
,92307932
,9283
,93166444
,93617630
,96024836
,99375910
|
|||||
| ChEBI ID |
ChEBI:6541
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km =100 microM | Chinese hamster ovary AA8 cells-MDR1 | [4] | |
| P-GP | Transporter Info | Km =232 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [2] | ||
| P-GP | Transporter Info | Km =403.2 microM | Madin-Darby canine kidney cells (MDCKII)-MDR1 | [2] | ||
| References | ||||||
| 1 | Losartan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Active transport of the angiotensin-II antagonist losartan and its main metabolite EXP 3174 across MDCK-MDR1 and caco-2 cell monolayers. Br J Pharmacol. 2000 Mar;129(6):1235-43. | |||||
| 3 | High-affinity interaction of sartans with H+/peptide transporters. Drug Metab Dispos. 2009 Jan;37(1):143-9. | |||||
| 4 | Competition of hydrophobic peptides, cytotoxic drugs, and chemosensitizers on a common P-glycoprotein pharmacophore as revealed by its ATPase activity. J Biol Chem. 1996 Feb 9;271(6):3163-71. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
