Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00399
|
|||||
| Drug Name |
Valproic Acid
|
|||||
| Synonyms |
2 PP (base); 2-Propylpentanoic acid; 2-Propylvaleric acid; 2-n-Propyl-n-valeric acid; 4-Heptanecarboxylic acid; Abbott 44090; Acide valproique; Acide valproique [INN-French]; Acido valproico; Acido valproico [INN-Spanish]; Acidum valproicum; Acidum valproicum [INN-Latin]; Alti-Valproic; Avugane; Baceca; Convulex; Convulex (TN); Convulsofin; Depacon (TN); Depakene; Depakene (TN); Depakin; Depakin chrono; Depakine; Depakine chrono; Depakote (TM); Depakote (TN); Depakote ER (TN); Deproic; Di-n-propylacetic acid; Di-n-propylessigsaeure; Di-n-propylessigsaure; Di-n-propylessigsaure [German]; Dipropyl Acetate; Dipropylacetate; Dipropylacetic acid; Dom-Valproic; Encorate (TN); Epilim (TN); Epival (TN); Ergenyl; G2M-777; Kyselina 2-propylvalerova; Kyselina 2-propylvalerova [Czech]; Med Valproic; Mylproin; Myproic Acid; N-DPA; N-Dipropylacetic acid; Novo-Valproic; Nu-Valproic; PMS-Valproic Acid; Penta-Valproic; Propylvaleric acid; Savicol; Semisodium Valproate; Stavzor; Stavzor (TN); VPA; Valproate; Valproic Acid, Sodium Salt (2:1); Valproic acid (USP); Valproic acid USP; Valproic acid USP24; Valproic acid [USAN:INN:BAN]; Valproinsaeure; Vupral; Winthrop (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Epilepsy [ICD11:8A6Z] | Approved | [1] | |||
| Therapeutic Class |
Anticonvulsants
|
|||||
| Structure |
|
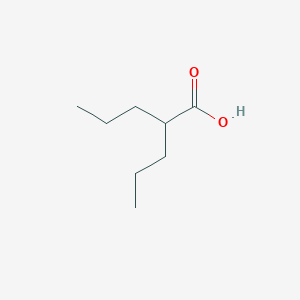 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H16O2
|
|||||
| Canonical SMILES |
CCCC(CCC)C(=O)O
|
|||||
| InChI |
InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)
|
|||||
| InChIKey |
NIJJYAXOARWZEE-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 99-66-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 144.21 | Topological Polar Surface Area | 37.3 | ||
| Heavy Atom Count | 10 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
2.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10539166
,11335448
,11360687
,11363415
,11364691
,11365977
,11367253
,11368539
,11369815
,11372725
,11372856
,11374010
,11375415
,11376701
,11377978
,11461659
,11484648
,11488762
,11491548
,11492191
,11494335
,12015354
,14710660
,15321539
,17389523
,24898751
,26697333
,26752920
,26752921
,29222263
,3138784
,399407
,46260925
,46505925
,48416692
,48424277
,48425730
,4918409
,49635685
,49640649
,49856166
,50062089
,50105536
,53788878
,56310655
,621684
,7847465
,7980632
,8151988
,9394
|
|||||
| ChEBI ID |
ChEBI:39867
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MCT2 | Transporter Info | Monocarboxylate transporter 2 | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| References | ||||||
| 1 | Valproic Acid was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Monocarboxylate Transporters in Drug Disposition: Role in the Toxicokinetics and Toxicodynamics of the Drug of Abuse GHB. | |||||
| 3 | Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010 Feb;44(2):152-67. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
