Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00313
|
|||||
| Drug Name |
Cefadroxil
|
|||||
| Synonyms |
(6R,7R)-7-((R)-2-Amino-2-(p-hydroxyphenyl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7beta-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3,4-didehydrocepham-4-carboxylic acid; BL-S 578; BL-S578; CDX; Cefadroxil (JP15); Cefadroxil anhydrous; Cefadroxilo; Cefadroxilo [INN-Spanish]; Cefadroxilum; Cefadroxilum [INN-Latin]; Cephadroxil; Curisafe (TN); D-Cefadroxil; MJF-11567-3; S 578; S-578; Sumacef; Sumacef (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gram-positive & negative bacteria infections [ICD11:1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
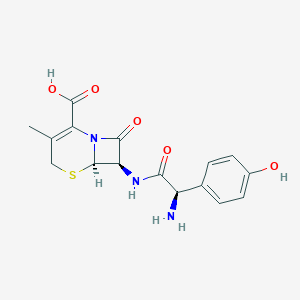 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H17N3O5S
|
|||||
| Canonical SMILES |
CC1=C(N2C(C(C2=O)NC(=O)C(C3=CC=C(C=C3)O)N)SC1)C(=O)O
|
|||||
| InChI |
InChI=1S/C16H17N3O5S/c1-7-6-25-15-11(14(22)19(15)12(7)16(23)24)18-13(21)10(17)8-2-4-9(20)5-3-8/h2-5,10-11,15,20H,6,17H2,1H3,(H,18,21)(H,23,24)/t10-,11-,15-/m1/s1
|
|||||
| InChIKey |
BOEGTKLJZSQCCD-UEKVPHQBSA-N
|
|||||
| CAS Number |
CAS 66592-87-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 363.4 | Topological Polar Surface Area | 158 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-2.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103620148
,103914339
,104133801
,104354273
,10990583
,11335584
,11360823
,11362958
,11365520
,11368082
,11373871
,11376244
,11461795
,11466462
,11467582
,11483750
,11486123
,11487903
,11492081
,11493918
,121363085
,124766005
,14852631
,16050996
,24892921
,25622160
,34712894
,46509128
,47440185
,47515253
,47515254
,47959665
,48035040
,48334422
,48415709
,49699087
,50124273
,57654040
,75439952
,7847323
,7978892
,8149235
,85279381
,87322627
,90451718
,9095
,92125414
,93576133
,93576712
,99301497
|
|||||
| ChEBI ID |
CHEBI:3479
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [2] | ||
| MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [2] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [2] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [3] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MRP1 | Transporter Info | Km =3.9 microM | Human embryonic kidney cells (HEK293)-MRP1 | [2] | |
| MRP3 | Transporter Info | Km =2.5 microM | Spodoptera frugiperda 21 (Sf21) cells-MRP3 | [2] | ||
| MRP4 | Transporter Info | Km =0.25 microM | Spodoptera frugiperda 21 (Sf21) cells-MRP4 | [2] | ||
| PEPT1 | Transporter Info | Km =7.97 microM | Human cervical cancer cell line (Hela)-PEPT1 | [3] | ||
| PEPT2 | Transporter Info | Km =150.8 microM | In vivo model (human) | [4] | ||
| References | ||||||
| 1 | Cefadroxil was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Oral availability of cefadroxil depends on ABCC3 and ABCC4. Drug Metab Dispos. 2012 Mar;40(3):515-21. | |||||
| 3 | High-affinity interaction of sartans with H+/peptide transporters. Drug Metab Dispos. 2009 Jan;37(1):143-9. | |||||
| 4 | Species Differences in Human and Rodent PEPT2-Mediated Transport of Glycylsarcosine and Cefadroxil in Pichia Pastoris Transformants. Drug Metab Dispos. 2017 Feb;45(2):130-136. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
