Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00287
|
|||||
| Drug Name |
Resveratrol
|
|||||
| Synonyms |
(E)-5-(2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol; (E)-5-(p-Hydroxystyryl)resorcinol; (E)-5-[2-(4-Hydroxyphenyl)ethenyl]-1,3-benzenediol; (E)-5-[2-(4-hydroxyphenyl)ethenyl]-1,3-benzendiol; (E)-resveratrol; 3,4',5-Stilbenetriol; 3,4',5-Trihydroxy-trans-stilbene; 3,4',5-Trihydroxystilbene; 3,4',5-trihydroxy-stilbene; 3,5,4'-Trihydroxy-trans-stilbene; 3,5,4'-Trihydroxystilbene; 5-((1E)-2-(4-Hydroxyphenyl)ethenyl)-1,3-benzenediol; 5-[(1E)-2-(4-Hydroxyphenyl)ethenyl]-1,3-benzenediol; 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol; 5-[(E)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol; 5-[2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol; CU-01000001503-3; Cis-resveratrol; KSC-10-164; KUC104385N; PREVENTION 8 (RESVERATROL); R 5010; RM-1812; Resveratrol-3-sulfate; Resvida; SRT 501; SRT-501; SRT501; Trans-1,2-(3,4',5-Trihydroxydiphenyl)ethylene; Trans-3,4′,5-Trihydroxystilbene; Trans-3,4',5-trihydroxystilbene; Trans-resveratrol
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Colorectal cancer [ICD11:2B91] | Discontinued in Phase 2 | [1] | |||
| Structure |
|
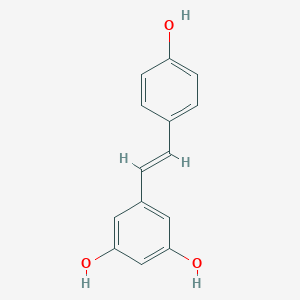 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C14H12O3
|
|||||
| Canonical SMILES |
C1=CC(=CC=C1C=CC2=CC(=CC(=C2)O)O)O
|
|||||
| InChI |
InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+
|
|||||
| InChIKey |
LUKBXSAWLPMMSZ-OWOJBTEDSA-N
|
|||||
| CAS Number |
CAS 375823-41-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 228.24 | Topological Polar Surface Area | 60.7 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
3.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10299643
,10321586
,11537676
,12015089
,14822845
,17388746
,17405667
,22388006
,24278055
,24715042
,24860876
,26512266
,26612338
,26679661
,26697115
,26736772
,26747076
,26747077
,26751586
,26751587
,26759440
,29204531
,36887952
,458391
,46386962
,46493745
,46504705
,47291249
,47515445
,47589112
,47959898
,48035263
,48035264
,48185110
,48185111
,48334633
,48424038
,48425593
,49661756
,49698529
,49734167
,49833229
,584207
,611168
,6374
,7890614
,8145576
,837578
,855986
,866574
|
|||||
| ChEBI ID |
CHEBI45713
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [3] | ||
| References | ||||||
| 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018787) | |||||
| 2 | The effect of low pH on breast cancer resistance protein (ABCG2)-mediated transport of methotrexate, 7-hydroxymethotrexate, methotrexate diglutamate, folic acid, mitoxantrone, topotecan, and resveratrol in in vitro drug transport models. Mol Pharmacol. 2007 Jan;71(1):240-9. | |||||
| 3 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
