Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00278
|
|||||
| Drug Name |
Famotidine
|
|||||
| Synonyms |
(1-Amino-3-(((2-((diaminomethylene)amino)-4-thiazolyl)methyl)thio)propylidene)sulfamide; (1Z)-3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-N'-sulfamoylpropanimidamide; (1Z)-N'-(aminosulfonyl)-3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)thio]propanimidamide; 3-(2-Guanidinothiazol-4-ylmethylthio)-N1-sulfamoylpropionamide; 3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-N-sulfamoylpropanimidamide; 3-[[2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methylsulfanyl]-N'-sulfamoylpro; 3-[[2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methylsulfanyl]-N'-sulfamoylpropanimidamide; Amfamox; Antodine; Apo-Famotidine; Apogastine; Bestidine; Blocacid; Brolin; Cepal; Confobos; Cronol; Cuantin; Dibrit 40; Digervin; Dinul; Dipsin; Dispromil; Dispronil; Duovel; Durater; Evatin; F 6889; F0530; FAMOTIDINE PRESERVATIVE FREE; FAMOTIDINE PRESERVATIVE FREE IN PLASTIC CONTAINER; Fadin; Fadine; Fadyn; Fagastine; Famo; Famocid; Famodar; Famodil; Famodin; Famodine; Famogard; Famonit; Famopsin; Famos; Famosan; Famotal; Famotep; Famotidina [Spanish]; Famotidine [USAN:BAN:INN:JAN]; Famotidinum [Latin]; Famotin; Famovane; Famowal; Famox; Famoxal; Famtac; Famulcer; Fanobel; Fanosin; Fanox; Farmotex; Ferotine; Fibonel; Fluxid; Fudone; Ganor; Gaster; Gastridan; Gastridin; Gastrion; Gastro; Gastrodomina; Gastrofam; Gastropen; Gastrosidin; HS-0054; Hacip; Huberdina; Ingastri; Invigan; L 643341; Lecedil; Logos; MK 208; MK-208; Mensoma; Midefam; Mosul; Motiax; Muclox; Mylanta AR; N'-(Aminosulfonyl)-3-([2-(diaminomethyleneamino)-4-thiazolyl]methylthio)propanamidine; Neocidine; Nevofam; Notidin; Novo-Famotidine; Nu-Famotidine; Nulceran; Nulcerin; PEPCID; PEPCID COMPLETE; PEPCID PRESERVATIVE FREE; PEPCID PRESERVATIVE FREE IN PLASTIC CONTAINER; Panalba; Pepcid (TN); Pepcid AC; Pepcid AC Gelcaps; PepcidRPD; Pepcidac; Pepcidin; Pepcidin Rapitab; Pepcidina; Pepcidine; Pepcidine (TN); Pepdif; Pepdine; Pepdul; Pepfamin; Peptan; Peptidin; Peptifam; Pepzan; Propanimidamide, 3-[[[2-[aminoiminomethyl)amino]-4-thiazoyl]methyl]thio]-N-(aminosulfonyl); Purifam; Quamatel; Quamtel; Renapepsa; Restadin; Rogasti; Rubacina; Sedanium-R; Sigafam; Supertidine; Tairal; Tamin; Tipodex; Topcid; Ulcatif; Ulceprax; Ulcofam; Ulfagel; Ulfam; Ulfamid; Ulfinol; Ulgarine; Vagostal; Weimok; Whitidin; YM 11170; YM-11170; YM-1170; Yamarin
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Peptic ulcer [ICD11:DA61] | Approved | [1] | |||
| Therapeutic Class |
Antiulcer Agents
|
|||||
| Structure |
|
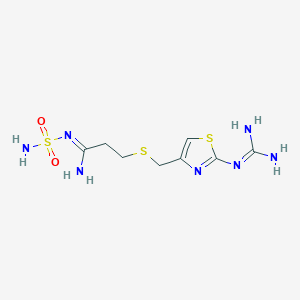 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H15N7O2S3
|
|||||
| Canonical SMILES |
C1=C(N=C(S1)N=C(N)N)CSCCC(=NS(=O)(=O)N)N
|
|||||
| InChI |
InChI=1S/C8H15N7O2S3/c9-6(15-20(12,16)17)1-2-18-3-5-4-19-8(13-5)14-7(10)11/h4H,1-3H2,(H2,9,15)(H2,12,16,17)(H4,10,11,13,14)
|
|||||
| InChIKey |
XUFQPHANEAPEMJ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 76824-35-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 337.5 | Topological Polar Surface Area | 238 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
-0.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321312
,11111194
,11111195
,11736775
,12013748
,14777800
,14900130
,14900131
,17405060
,219459
,24278442
,26719681
,26753575
,46386903
,47350438
,47425376
,47721689
,47957418
,48108291
,48244468
,48407560
,48415998
,49681730
,49698346
,49835099
,49846705
,50022089
,50100994
,50100995
,50106279
,50106280
,53777603
,57364911
,76715592
,77320398
,7847384
,7979217
,81093272
,85211955
,85220228
,85231052
,855518
,87558227
,90340682
,90501992
,91704175
,92125054
,92303791
,92308237
,92308848
|
|||||
| ChEBI ID |
ChEBI:4975
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 2-Oct | Transporter Info | Organic cation transporter 2 | Substrate | [2] | |
| OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [3] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [2] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | 2-Oct | Transporter Info | Km =56.1 microM | Human embryonic kidney cells (HEK293)-OCT2 | [2] | |
| OAT3 | Transporter Info | Km =124 microM | Human embryonic kidney cells (HEK293)-OAT3 | [4] | ||
| References | ||||||
| 1 | Famotidine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther. 2005 Oct;315(1):337-45. | |||||
| 3 | Different transport properties between famotidine and cimetidine by human renal organic ion transporters (SLC22A). Eur J Pharmacol. 2004 Oct 25;503(1-3):25-30. | |||||
| 4 | Is the monkey an appropriate animal model to examine drug-drug interactions involving renal clearance? Effect of probenecid on the renal elimination of H2 receptor antagonists. J Pharmacol Exp Ther. 2006 Mar;316(3):1187-94. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
