Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00270
|
|||||
| Drug Name |
Vigabatrin
|
|||||
| Synonyms |
(R,S)-4-Amino-5-hexenoic acid; (inverted question mark)-gamma-Vinyl GABA; 4-Amino-5-hexenoic acid; 4-Aminohexenoic acid; 4-aminohex-5-enoic acid; Acid, gamma-Vinyl-gamma-Aminobutyric; Aventis Brand of Vigabatrin; CPP-109; GVG; Gamma Vinyl GABA; Gamma Vinyl gamma Aminobutyric Acid; Gamma-Vinyl GABA; Gamma-Vinyl-GABA; Gamma-Vinyl-gamma-Aminobutyric Acid; Hexenoic acid, 4-amino; Hoechst Brand of Vigabatrin; M071754; MDL 71,754; MDL 71754; MDL-71754; RMI 71754; RMI-71754; RMI-71890; Sabril; Sabril (TN); Sabrilex; Sabrilex (TN); V 8261; V8261_SIGMA; Vigabatrin (JAN/USAN/INN); Vigabatrin Aventis Brand; Vigabatrin Hoechst Brand; Vigabatrin Yamanouchi Brand; Vigabatrin [USAN:BAN:INN]; Vigabatrin [USAN:INN:BAN]; Vigabatrina; Vigabatrina [Spanish]; Vigabatrine; Vigabatrine [French]; Vigabatrinum; Vigabatrinum [Latin]; Yamanouchi Brand of Vigabatrin
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Complex partial seizure [ICD11:8A6Z] | Approved | [1] | |||
| Epilepsy [ICD11:8A6Z] | Approved | [1] | ||||
| Infantile spasm [ICD11:8A62.0] | Approved | [1] | ||||
| Therapeutic Class |
Anticonvulsants
|
|||||
| Structure |
|
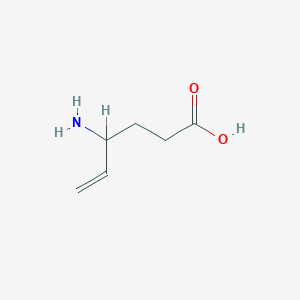 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C6H11NO2
|
|||||
| Canonical SMILES |
C=CC(CCC(=O)O)N
|
|||||
| InChI |
InChI=1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9)
|
|||||
| InChIKey |
PJDFLNIOAUIZSL-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 60643-86-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 129.16 | Topological Polar Surface Area | 63.3 | ||
| Heavy Atom Count | 9 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-2.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321780
,11341935
,11362118
,11363262
,11365824
,11368386
,11373068
,11376548
,11466529
,11467649
,11486078
,11487520
,11491640
,11494182
,12012774
,15194489
,17405831
,24278202
,26612272
,26747129
,26751903
,29224702
,46507052
,47217077
,47662561
,47960037
,47960038
,47960039
,48035425
,48334803
,48334804
,49698839
,49976606
,50104581
,50104582
,50104583
,53778383
,53788300
,5440157
,56313009
,57322887
,634838
,7847601
,79363997
,7980880
,8153478
,85231284
,90340732
,92125387
,9703
|
|||||
| ChEBI ID |
CHEBI:63638
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PAT1 | Transporter Info | Proton-coupled amino acid transporter 1 | Substrate | [2] | |
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | PAT1 | Transporter Info | Km =5200 microM | X.Laevis oocytes-hPAT1 | [2] | |
| References | ||||||
| 1 | Vigabatrin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Rectal absorption of vigabatrin, a substrate of the proton coupled amino acid transporter (PAT1, Slc36a1), in rats. Pharm Res. 2012 Apr;29(4):1134-42. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
