Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00263
|
|||||
| Drug Name |
Nizatidine
|
|||||
| Synonyms |
(E)-1-N'-[2-[[2-(dimethylaminomethyl)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine; (E)-N-[2-[[2-(dimethylaminomethyl)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-N'-methyl-2-nitroethene-1,1-diamine; (E)-N-{2-[({2-[(dimethylamino)methyl]-1,3-thiazol-4-yl}methyl)thio]ethyl}-N'-methyl-2-nitroethene-1,1-diamine; Acinon; Acinon (TN); Antizid; Axid; Axid (TN); Axid Ar; Calmaxid; Cronizat; Distaxid; Galitidin; Gastrax; LY 139037; LY-139037; N-(2-(((2-((Dimethylamino)methyl)-4-thiazolyl)methyl)thio)ethyl)-N'-methyl-2-nitro-1,1-ethenediamine; N-(4-(6-Methylamino-7-nitro-2-thia-5-aza-6-hepten-1-yl)-2-thiazolylmethyl)-N,N-dimethylamin; Naxidine; Niatidine; Nizatidina; Nizatidina [Spanish]; Nizatidine (JAN/USP/INN); Nizatidine [USAN:BAN:INN:JAN]; Nizatidinum; Nizatidinum [Latin]; Nizax; Nizaxid; Panaxid; Splendil ER; Tazac; Tazac (TN); Ulcosol; Ulxid; ZE-101; ZL-101; Zanizal; Zinga
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acid reflux disorder [ICD11:DA22] | Approved | [1] | |||
| Therapeutic Class |
Antiulcer Agents
|
|||||
| Structure |
|
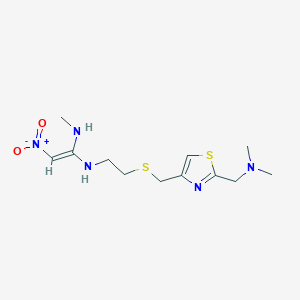 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C12H21N5O2S2
|
|||||
| Canonical SMILES |
CNC(=C[N+](=O)[O-])NCCSCC1=CSC(=N1)CN(C)C
|
|||||
| InChI |
InChI=1S/C12H21N5O2S2/c1-13-11(6-17(18)19)14-4-5-20-8-10-9-21-12(15-10)7-16(2)3/h6,9,13-14H,4-5,7-8H2,1-3H3/b11-6+
|
|||||
| InChIKey |
SGXXNSQHWDMGGP-IZZDOVSWSA-N
|
|||||
| CAS Number |
CAS 76963-41-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 331.5 | Topological Polar Surface Area | 140 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
1.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10047729
,103091650
,103157346
,103189241
,11112849
,111634652
,117377111
,117480196
,119526527
,12012865
,124658842
,124801333
,124882685
,126630925
,131269073
,131326051
,134337651
,135011516
,135692320
,137005454
,142971063
,144075999
,144089140
,14801956
,152040062
,160963930
,172080209
,177748315
,178103822
,179116550
,179148990
,26719892
,36077514
,46386765
,46507554
,48395159
,48416335
,49648463
,49968698
,57352747
,7847506
,7980136
,80039644
,85175204
,85209480
,92308159
,92308434
,92711372
,93166919
,9479
|
|||||
| ChEBI ID |
ChEBI:7601
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Nizatidine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 3 | DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB00585) | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
