Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00259
|
|||||
| Drug Name |
Cefaclor
|
|||||
| Synonyms |
(6R,7R)-7-((R)-2-Amino-2-phenylacetamido)-3-chloro-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid monohydrate; (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid hydrate; (6R,7R)-7-{[(2R)-2-amino-2-phenylacetyl]amino}-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 3-Chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid; 3-Chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate; 7-(2-Amino-2-phenyl-acetylamino)-3-chloro-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7beta-{[(2R)-2-amino-2-phenylacetyl]amino}-3-chloro-3,4-didehydrocepham-4-carboxylic acid; Alenfral; Alenfral (TN); Alfacet; Alfatil; Alfatil Kapseln; CCL; Ceclor; Ceclor (TN); Ceclor, Distaclor, Keflor, Raniclor, Cefaclor; Cefaclor (JP15); Cefaclor (USP); Cefaclor [USAN:INN:BAN:JAN]; Cefaclor anhydrous; Cefaclor hydrate; Cefaclor monohydrate; Cefaclor-1-wasser; Cefaclorum; Distaclor; Distaclor (TN); Dystaclor MR; Keflor (TN); Kefolor; Kefolor Suspension; L-Kefral; Lilly 99638 hydrate; Muco Panoral; Panacef; Panoral; Raniclor (TN); S-6472
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gram-positive & negative bacteria infections [ICD11:1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
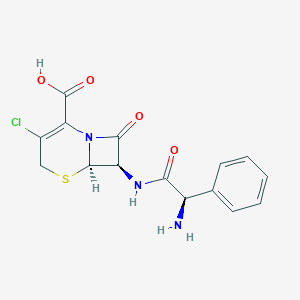
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H14ClN3O4S
|
|||||
| Canonical SMILES |
C1C(=C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=CC=C3)N)C(=O)O)Cl
|
|||||
| InChI |
InChI=1S/C15H14ClN3O4S/c16-8-6-24-14-10(13(21)19(14)11(8)15(22)23)18-12(20)9(17)7-4-2-1-3-5-7/h1-5,9-10,14H,6,17H2,(H,18,20)(H,22,23)/t9-,10-,14-/m1/s1
|
|||||
| InChIKey |
QYIYFLOTGYLRGG-GPCCPHFNSA-N
|
|||||
| CAS Number |
CAS 53994-73-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 367.8 | Topological Polar Surface Area | 138 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
-1.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11342218
,11362401
,11363964
,11366526
,11369088
,11372875
,11373525
,11377250
,11466513
,11467130
,11467633
,11468250
,11484973
,11486006
,11486818
,11487803
,11489005
,11491673
,11491908
,11494884
,12013565
,14803830
,14852885
,24278331
,26612067
,26680440
,29215008
,34715524
,46386958
,46507693
,47425324
,47425325
,47574401
,47647541
,47796074
,48020027
,48095565
,48170475
,48244405
,48244406
,48415708
,48484011
,49681804
,49698517
,625389
,7847322
,7978871
,8181891
,855765
,9094
|
|||||
| ChEBI ID |
CHEBI:3478
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | |
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [3] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [4] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | PEPT2 | Transporter Info | Km =72 microM | Madin-Darby canine kidney (MDCK) cells-PEPT2 | [5] | |
| References | ||||||
| 1 | Cefaclor was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | FDA Drug Development and Drug Interactions | |||||
| 3 | Human organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1. Biochem Pharmacol. 2005 Oct 1;70(7):1104-13. | |||||
| 4 | Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am J Physiol Gastrointest Liver Physiol. 2011 May;300(5):G895-902. | |||||
| 5 | Interactions of amoxicillin and cefaclor with human renal organic anion and peptide transporters. Drug Metab Dispos. 2006 Apr;34(4):547-55. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
