Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00232
|
|||||
| Drug Name |
Probenecid
|
|||||
| Synonyms |
4-((Dipropylamino)sulfonyl)benzoic acid;4-(Di-n-propylsulfamoyl)benzoesaeure; 4-(Dipropylsulfamoyl)benzoic acid; 4-(N,N-Dipropylsulfamoyl)benzoesaeure; 4-[(dipropylamino)sulfonyl]benzoic acid; Apurina; Bencid; Benecid; Benemid; Benemid (TN); Benemide; Benuryl; Benuryl (TN); Biokanol Brand of Probenecid; Col-BENEMID; ColBenemid (co mponent of); ColBenemid (component of); ICN Brand of Probenecid; IDIS Brand of Probenecid; Major Brand of Probenecid; Martec Brand of Probenecid; Merck Brand of Probenecid; Ophthalmic Brand of Probenecid; P-(Dipropylsulfamoyl)benzoic acid; P-(Dipropylsulfamyl)benzoic acid; P-[Dipropylsulfamoyl]benzoic acid; Panuric; Parabenem; Parmed Brand of Probenecid; Polycillin-BRB; Polycillin-PRB (component of); Pro-Cid; Probalan; Probampacin; Probecid; Proben; Probenecid (JP15/USP/INN); Probenecid Major Brand; Probenecid Martec Brand; Probenecid Parmed Brand; Probenecid Weimer; Probenecid Zenith Brand; Probenecid [INN:BAN:JAN]; Probenecid acid; Probenecida; Probenecida [INN-Spanish]; Probenecide; Probenecide [INN-French]; Probenecidum; Probenecidum [INN-Latin]; Probenemid; Probenicid; Probenid; Probexin; Prolongine; Robenecid; Sulprin; Synergid R; Tubophan; Uricosid; Urocid; Valdecasas Brand of Probenecid; Zenith Brand of Probenecid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gout [ICD11:FA25] | Approved | [1] | |||
| Hyperuricaemia [ICD11:5C55.Y] | Approved | [1] | ||||
| Therapeutic Class |
Uricosuric Agents
|
|||||
| Structure |
|
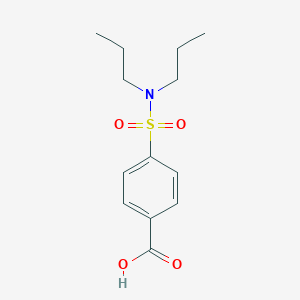 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C13H19NO4S
|
|||||
| Canonical SMILES |
CCCN(CCC)S(=O)(=O)C1=CC=C(C=C1)C(=O)O
|
|||||
| InChI |
InChI=1S/C13H19NO4S/c1-3-9-14(10-4-2)19(17,18)12-7-5-11(6-8-12)13(15)16/h5-8H,3-4,9-10H2,1-2H3,(H,15,16)
|
|||||
| InChIKey |
DBABZHXKTCFAPX-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 57-66-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 285.36 | Topological Polar Surface Area | 83.1 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
3.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321755
,10528000
,11112135
,11335231
,11360470
,11363728
,11366290
,11368852
,11371827
,11374110
,11377014
,11461442
,11466570
,11467690
,11485063
,11486240
,11489110
,11490370
,11492301
,11494648
,14873183
,17389752
,24898976
,26611889
,26680013
,26747055
,26747056
,26751571
,29223989
,3162120
,46506554
,47588826
,47662104
,47885248
,47959564
,47959565
,48034940
,48110289
,48334309
,48414371
,48416470
,48423998
,5227618
,7847541
,7980385
,8149496
,8153022
,81789
,855953
,9576
|
|||||
| ChEBI ID |
CHEBI:8426
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MCT6 | Transporter Info | Monocarboxylate transporter 6 | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [4] | ||
| References | ||||||
| 1 | Probenecid was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Quercetin, Morin, Luteolin, and Phloretin Are Dietary Flavonoid Inhibitors of Monocarboxylate Transporter 6. Mol Pharm. 2017 Sep 5;14(9):2930-2936. | |||||
| 3 | MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer. 2005 Sep 20;116(5):824-9. | |||||
| 4 | Characterization of the efflux transport of 17beta-estradiol-D-17beta-glucuronide from the brain across the blood-brain barrier. J Pharmacol Exp Ther. 2001 Jul;298(1):316-22. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
