Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00217
|
|||||
| Drug Name |
Gabapentin
|
|||||
| Synonyms |
1-(Aminomethyl)-cyclohexaneacetic acid; 1-(Aminomethyl)cyclohexaneacetic acid; 2-[1-(aminomethyl)cyclohexyl]acetic acid; Aclonium; Apo-Gabapentin; Apotex brand of gabapentin; Aventis brand of gabapentin; DDS-2003; DM-1796; DM-5689; G-154; GBN; GOE 2450; Gabapen; Gabapentin (JAN/USAN/INN); Gabapentin (Neurontin); Gabapentin GR; Gabapentin Hexal; Gabapentin Stada; Gabapentin [USAN:INN:BAN]; Gabapentin-ratiopharm; Gabapentina; Gabapentine; Gabapentine [INN-French]; Gabapentinium; Gabapentino; Gabapentino [INN-Spanish]; Gabapentino [Spanish]; Gabapentinum; Gabapentinum [INN-Latin]; Gabapetin; Go 3450; Goe-3450; Hexal brand of gabapentin; Neurontin; Neurontin (TN); Novo-Gabapentin; Novopharm brand of gabapentin; PMS-Gabapentin; Parke Davis brand of gabapentin; Pfizer brand of gabapentin; Pharmascience brand of gabapentin; Ratiopharm brand of gabapentin; Serada; Stadapharm brand of gabapentin; Vultin; Warner-Lambert brand of gabapentin; [1-(AMINOMETHYL)CYCLOHEXYL]ACETIC ACID
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Partial seizures [ICD11:8A68.0] | Approved | [1] | |||
| Therapeutic Class |
Analgesics
|
|||||
| Structure |
|
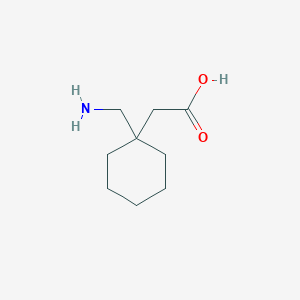 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C9H17NO2
|
|||||
| Canonical SMILES |
C1CCC(CC1)(CC(=O)O)CN
|
|||||
| InChI |
InChI=1S/C9H17NO2/c10-7-9(6-8(11)12)4-2-1-3-5-9/h1-7,10H2,(H,11,12)
|
|||||
| InChIKey |
UGJMXCAKCUNAIE-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 60142-96-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 171.24 | Topological Polar Surface Area | 63.3 | ||
| Heavy Atom Count | 12 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-1.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321252
,11111218
,11112806
,11113327
,11466889
,11468009
,11486558
,11528594
,12012796
,15171186
,17405144
,24278159
,25623436
,29222580
,3206533
,46506529
,47291223
,47365305
,47515412
,47589087
,48035236
,48413201
,48416046
,49655262
,49698669
,49833701
,50100248
,50103936
,50103937
,50519620
,53777688
,56312982
,56365854
,5651496
,57321804
,7847398
,7887769
,7979342
,8028266
,8028269
,8152192
,85174424
,85209452
,85231060
,855579
,87560508
,89649772
,90340599
,91146476
,92125867
|
|||||
| ChEBI ID |
ChEBI:42797
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 2-Oct | Transporter Info | Organic cation transporter 2 | Substrate | [2] | |
| LAT1 | Transporter Info | L-type amino acid transporter 1 | Substrate | [3] | ||
| OCTN1 | Transporter Info | Organic cation/carnitine transporter 1 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | LAT1 | Transporter Info | Km =530 microM | hCMEC/D3 cells-LAT1 | [3] | |
| References | ||||||
| 1 | Gabapentin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Clinical pharmacokinetic drug interaction studies of gabapentin enacarbil, a novel transported prodrug of gabapentin, with naproxen and cimetidine. Br J Clin Pharmacol. 2010 May;69(5):498-507. | |||||
| 3 | Transport of gabapentin by LAT1 (SLC7A5). Biochem Pharmacol. 2013 Jun 1;85(11):1672-83. | |||||
| 4 | Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin Pharmacol Ther. 2008 Mar;83(3):416-21. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
