Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00190
|
|||||
| Drug Name |
Amoxicillin
|
|||||
| Synonyms |
(-)-6-(2-Amino-2-(P-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo-(3.2.0)heptane-2-carboxylic acid; (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(2-amino-2-(p-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-, D-(8CI); 6-(D-(-)-alpha-Amino-p-hydroxyphenylacetamido)penicillanic acid; 6-(D-(-)-p-Hydroxy-alpha-aminobenzyl)penicillin; 6-(p-Hydroxy-alpha-aminophenylacetamido)penicillanic acid; 6beta-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-2,2-dimethylpenam-3alpha-carbonyl; 6beta-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-2,2-dimethylpenam-3alpha-carboxylic acid; AMK (TN); AMOXICILLIN CRYSTALLINE; AMOXICILLIN PEDIATRIC; AMPC; Actimoxi; Actimoxi (TN); Alpha-Amino-p-hydroxybenzylpenicillin; Alphamox (TN); Amoclen; Amoksibos (TN); Amoksiklav (TN); Amolin; Amopen; Amopenixin; Amoxi; Amoxi-Mast; Amoxibiotic; Amoxibiotic (TN); Amoxicaps; Amoxicilina; Amoxicilina (TN); Amoxicilina [INN-Spanish]; Amoxicillanyl; Amoxicillin (INN); Amoxicillin (TN); Amoxicillin (anhydrous); Amoxicillin anhydrous; Amoxicilline; Amoxicilline [INN-French]; Amoxicilline [INN]; Amoxicillinum; Amoxicillinum [INN-Latin]; Amoxiclav (TN); Amoxidal (TN); Amoxiden; Amoxil; Amoxil (TN); Amoxin (TN); Amoxivet; Amoxycillin; Amoxycillin Trihydrate; Anemolin; Apo-Amoxi; Apo-Amoxi (TN); Aspenil; Augmentin (TN); BL-P 1410; BLP 1410; BRL-2333; Bactox (TN); Betalaktam (TN); Biomox; Bristamox; Cemoxin; Cilamox (TN); Clamoxyl; Clamoxyl (TN); Curam (TN); D-(-)-alpha-Amino-p-hydroxybenzylpenicillin; D-2-Amino-2-(4-hydroxyphenyl)acetamidopenicillanic acid; D-Amoxicillin; Dedoxil (TN); Delacillin; DisperMox; Dispermox (TN); Duomox (TN); Efpenix; Enhancin (TN); Flemoxin; Geramox (TN); Gimalxina (TN); Hiconcil; Hiconcil (TN); Histocillin; Hydroxyampicillin; Ibiamox; Imacillin; Isimoxin (TN); Klavox (TN); Lamoxy; Lamoxy (TN); Larotid; Metafarma capsules; Metifarma capsules; Moxacin; Moxal; Moxatag; Moxatag (TN); Moxilen (TN); Moxypen (TN); Moxyvit (TN); Nobactam (TN); Novamoxin (TN); Ospamox; Ospamox (TN); P-Hydroxyampicillin; Pamoxicillin; Pamoxicillin (TN); Panamox (TN); Panklav (TN); Piramox; Polymox; Polymox (TN); Ro 10-8756; Robamox; Samthongcillin (TN); Sandoz (TN); Sawamox PM; Senox (TN); Sinacilin (TN); Sumox; Tolodina; Tolodina (TN); Trimox; Trimox (TN); Unicillin; Utimox; Vetramox; Wymox; Wymox (TN); Yucla (TN); Zerrsox (TN); Zimox (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Streptococcal pharyngitis [ICD11:1B51] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
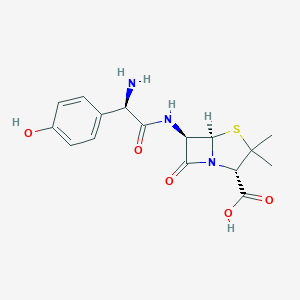 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H19N3O5S
|
|||||
| Canonical SMILES |
CC1(C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=C(C=C3)O)N)C(=O)O)C
|
|||||
| InChI |
InChI=1S/C16H19N3O5S/c1-16(2)11(15(23)24)19-13(22)10(14(19)25-16)18-12(21)9(17)7-3-5-8(20)6-4-7/h3-6,9-11,14,20H,17H2,1-2H3,(H,18,21)(H,23,24)/t9-,10-,11+,14-/m1/s1
|
|||||
| InChIKey |
LSQZJLSUYDQPKJ-NJBDSQKTSA-N
|
|||||
| CAS Number |
CAS 26787-78-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 365.4 | Topological Polar Surface Area | 158 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321668
,103296891
,103914155
,104170168
,104178892
,104316487
,11466385
,11467505
,11486110
,119525230
,121362635
,121363090
,124766400
,124799877
,126630772
,126657267
,134337739
,134997708
,135769296
,136357150
,137002906
,139157548
,14803689
,14852747
,34675405
,46507578
,47277019
,47500930
,48020429
,48244812
,49698452
,50050650
,50124271
,51091783
,56310905
,56312972
,56464141
,57288747
,57311544
,625395
,75691305
,7978697
,8173210
,85148361
,855939
,85787504
,9045
,92125419
,92711519
,96099939
|
|||||
| ChEBI ID |
ChEBI:2676
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [4] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | PEPT2 | Transporter Info | Km =1040 microM | Madin-Darby canine kidney (MDCK) cells-PEPT2 | [4] | |
| References | ||||||
| 1 | Pantoprazole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion-correlation of in vivo and in vitro studies. Drug Metab Dispos. 2002 Jan;30(1):13-9. | |||||
| 3 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
| 4 | Interactions of amoxicillin and cefaclor with human renal organic anion and peptide transporters. Drug Metab Dispos. 2006 Apr;34(4):547-55. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
