Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00175
|
|||||
| Drug Name |
Zalcitabine
|
|||||
| Synonyms |
1-(2,3-Dideoxy-beta-D-ribofuranosyl)cytosine; 2',3'-DIDEOXYCYTIDINE; 2',3'-Dideoxycytidine & Interferon-alpha; 2',3'-Dideoxycytidine & Nanoparticles (from human serum albumin or polyhexylcyanoacrylate); 2',3'-Dideoxycytidine & sCD4(soluble recombinant protein); 2,3-dideoxycytidine; 3'-Azido-3'-deoxythymidine/2',3'-Dideoxycytidine; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one; Beta-D-2',3'-Dideoxycytidine; Beta-D-2',3'-Dideoxycytidine & Granulocyte-macrophage colony-stimulating factor; Beta-D-DDC; Cytidine, 2',3'-dideoxy & Interferon alpha; Cytidine, 2',3'-dideoxy-& Colony-stimulating factor; D 5782; DDC (DDC); DS-4152 & ddC; DdC; DdC & GM-CSF; DdC & IFN-alpha; DdC & Interferon alpha; DdC & NP (from PHCA or HSA); DdC & sCD4; DdC (Antiviral); DdCyd; Dideoxycytidine; HIVID; Hivid (TN); Hivid(TM); Hivid, Dideoxycytidine, NSC 606170, Zalcitabine; Interferon AD + ddC; KS-1130; Lecithinized superoxide dismutase & .beta.-D-2',3'-Dideoxycytidine; PC-SOD & ddC; Ro 24-2027/000; Ro-24-2027/000; SRI-7707; Sulfated polysaccharide-peptidoglycan DS-4152 & 2',3'-Dideoxycytidine; Zalcitabine (JAN/USP/INN); Zalcitabine [USAN:INN:BAN]; Zalcitibine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Human immunodeficiency virus infection [ICD11:1C62.Z] | Approved | [1] | |||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
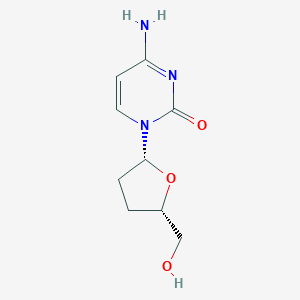
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C9H13N3O3
|
|||||
| Canonical SMILES |
C1CC(OC1CO)N2C=CC(=NC2=O)N
|
|||||
| InChI |
InChI=1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1
|
|||||
| InChIKey |
WREGKURFCTUGRC-POYBYMJQSA-N
|
|||||
| CAS Number |
CAS 7481-89-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 211.22 | Topological Polar Surface Area | 88.2 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-1.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11467065
,11468185
,11486840
,11504751
,11528334
,11532889
,14797711
,15171927
,17389522
,17404923
,22391526
,22431601
,24278377
,24862834
,25819962
,26719633
,26752752
,26752753
,29291163
,3259589
,46386814
,46488030
,46507879
,47216920
,47216921
,47589142
,48185139
,48334652
,48416706
,48422195
,48422429
,48423540
,48424478
,49681781
,49693297
,49699414
,49734166
,50105404
,50105405
,595891
,596441
,596607
,596679
,601537
,602107
,7847478
,7980904
,8167576
,878108
,9416
|
|||||
| ChEBI ID |
CHEBI:10101
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP8 | Transporter Info | Multidrug resistance-associated protein 8 | Substrate | [2] | |
| OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Zalcitabine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2',3'-dideoxycytidine and 9'-(2'-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003 Aug 8;278(32):29509-14. | |||||
| 3 | Interaction of zalcitabine with human organic anion transporter 1. Pharmazie. 2006 May;61(5):491-2. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
