Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00153
|
|||||
| Drug Name |
Fluorouracil
|
|||||
| Synonyms |
1-fluoro-1h-pyrimidine-2,4-dione; 2,4-Dihydroxy-5-fluoropyrimidine; 2,4-Dioxo-5-fluoropryimidine; 2,4-Dioxo-5-fluoropyrimidine; 5 FU Lederle; 5 FU medac; 5 Fluorouracil; 5 Fluorouracil biosyn; 5 HU Hexal; 5-FU; 5-FU (TN); 5-FU Lederle; 5-FU medac; 5-Faracil; 5-Fluor-2,4(1H,3H)-pyrimidindion; 5-Fluor-2,4(1H,3H)-pyrimidindion [Czech]; 5-Fluor-2,4-dihydroxypyrimidin; 5-Fluor-2,4-dihydroxypyrimidin [Czech]; 5-Fluor-2,4-pyrimidindiol; 5-Fluor-2,4-pyrimidindiol [Czech]; 5-Fluoracil; 5-Fluoracil [German]; 5-Fluoracyl; 5-Fluoro-2,4(1H,3H)-pyrimidinedione; 5-Fluoro-2,4-pyrimidinedione; 5-Fluoropyrimidin-2,4-diol; 5-Fluoropyrimidine-2,4-dione; 5-Fluorouracil; 5-Fluorouracil-biosyn; 5-Fluoruracil; 5-Fluoruracil [German]; 5-Ftouracyl; 5-HU Hexal; 5-fluoro uracil; 5-fluoro-1H-pyrimidine-2,4-dione; 5-fluoropyrimidine-2,4(1H,3H)-dione; 5FU; Adrucil; Adrucil (TN); Allergan Brand of Fluorouracil; Arumel; Biosyn Brand of Fluorouracil; CSP Brand of Fluorouracil; Carac; Carac (TN); Carzonal; Cinco FU; Dakota Brand of Fluorouracil; Dakota, Fluorouracile; Dermatech Brand of Fluorouracil; Dermik Brandof Fluorouracil; Effluderm; Effluderm (free base); Efudex; Efudex (TN); Efudix; Efurix; F 6627; F0151; Ferrer Brand of Fluorouracil; Fluoro Uracile ICN; Fluoro-Uracile ICN; Fluoro-uracile; Fluoro-uracilo; Fluoroblastin; Fluoroplex; Fluoroplex (TN); Fluorouracil (JP15/USP/INN); Fluorouracil GRY; Fluorouracil Mononitrate; Fluorouracil Monopotassium Salt; Fluorouracil Monosodium Salt; Fluorouracil Potassium Salt; Fluorouracil Teva Brand; Fluorouracil [USAN:INN:BAN:JAN]; Fluorouracil-GRY; Fluorouracile; Fluorouracile Dakota; Fluorouracile [DCIT]; Fluorouracilo; Fluorouracilo Ferrer Far; Fluorouracilo [INN-Spanish]; Fluorouracilum; Fluorouracilum [INN-Latin]; Fluoruracil; Fluracedyl; Fluracil; Fluracilum; Fluri; Fluril; Fluro Uracil; Fluroblastin; Flurodex; Ftoruracil; Gry Brand of Fluorouracil; Haemato Brand of Fluorouracil; Haemato fu; Haemato-fu; Hexal Brand of Fluorouracil; ICN Brand of Fluorouracil; IN1335; Inhibits thymilidate synthetase; Kecimeton; Medac Brand of Fluorouracil; Neocorp Brand of Fluorouracil; Neofluor; Onkofluor; Onkoworks Brand of Fluorouracil; Phthoruracil; Phtoruracil; Queroplex; Ribofluor; Ribosepharm Brand of Fluorouracil; Riemser Brand of Fluorouracil; Ro 2-9757; Ro-2-9757; Roche Brand of Fluorouracil; Tetratogen; Teva Brand of Fluorouracil; Timazin; U 8953; U-8953; URF; Ulup
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Colon cancer [ICD11:2B90.Z] | Approved | [1] | |||
| Esophageal cancer [ICD11:2B70] | Approved | [1] | ||||
| Stomach cancer [ICD11:2B72] | Approved | [1] | ||||
| Pancreatic cancer [ICD11:2C10] | Approved | [1] | ||||
| Therapeutic Class |
Immunosuppressive Agents
|
|||||
| Structure |
|
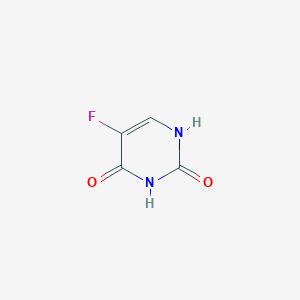 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C4H3FN2O2
|
|||||
| Canonical SMILES |
C1=C(C(=O)NC(=O)N1)F
|
|||||
| InChI |
InChI=1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
|
|||||
| InChIKey |
GHASVSINZRGABV-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 51-21-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 130.08 | Topological Polar Surface Area | 58.2 | ||
| Heavy Atom Count | 9 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10524722
,11111190
,11335229
,11360468
,11363735
,11366297
,11368859
,11371368
,11374392
,11377021
,11406045
,11461440
,11484027
,11487892
,11490250
,11492372
,11494655
,11538022
,15218968
,17389875
,17405099
,22391543
,24276773
,24278439
,24871165
,24894963
,25346604
,25621761
,26611750
,26679238
,26697058
,26747342
,26747343
,26747344
,26752979
,26758708
,3139714
,5132902
,5367838
,595836
,603131
,7847650
,7891022
,7978600
,8139872
,8149350
,8152156
,82653
,841046
,9851
|
|||||
| ChEBI ID |
ChEBI:46345
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| ENT1 | Transporter Info | Equilibrative nucleoside transporter 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [5] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [5] | ||
| MRP5 | Transporter Info | Multidrug resistance-associated protein 5 | Substrate | [5] | ||
| OAT2 | Transporter Info | Organic anion transporter 2 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [7] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OAT2 | Transporter Info | Km =0.0538 microM | Oocytes-OAT2 | [6] | |
| References | ||||||
| 1 | Fluorouracil was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Role of BCRP as a biomarker for predicting resistance to 5-fluorouracil in breast cancer. Cancer Chemother Pharmacol. 2009 May;63(6):1103-10. | |||||
| 3 | Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res. 2007 Jul-Aug;27(4B):2241-9. | |||||
| 4 | Enhancing chemosensitivity in oral squamous cell carcinoma by lentivirus vector-mediated RNA interference targeting EGFR and MRP2. Oncol Lett. 2016 Sep;12(3):2107-2114. | |||||
| 5 | ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology. 2009;9(1-2):136-44. | |||||
| 6 | Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005 May;57(5):573-8. | |||||
| 7 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
