Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00140
|
|||||
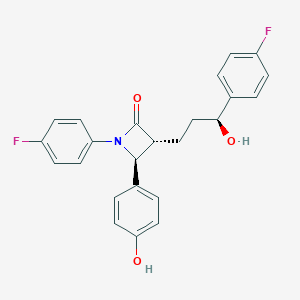
| Drug Name |
Ezetimibe
|
|||||
| Synonyms |
(-)-Sch 58235; (1-(4-fluorophenyl)-(3R)-(3-(4-fluorophenyl)-(3S)-hydroxypropyl)-(4S)-(4-hydroxyphenyl)-2-azetidinone); (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one; (3R,4S)-1-(p-Fluorophenyl)-3-((3S)-3-(p-fluorophenyl)-3-hydroxypropyl)-4-(p-hydroxyphenyl)-2-azetidinone; 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-(4-hydroxyphenyl)-2-azetidione; 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone; Essex brand of ezetimibe; Ezedoc; Ezetimib; Ezetimibe (JAN/USAN/INN); Ezetimibe [USAN:INN]; Ezetrol; Inegy (TN); MK-0653; MSD brand of ezetimibe; Merck brand of ezetimibe; SCH-58235; SCH58235; Sch 58235; Schering-Plough brand of ezetimibe; Vytorin (TN); Zetia; Zetia (TN); Zetia , Ezetrol, Ezetimibe; Zient
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hypercholesterolemia [ICD11:5C80.0] | Approved | [1] | |||
| Therapeutic Class |
Anticholesteremic Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H21F2NO3
|
|||||
| Canonical SMILES |
C1=CC(=CC=C1C2C(C(=O)N2C3=CC=C(C=C3)F)CCC(C4=CC=C(C=C4)F)O)O
|
|||||
| InChI |
InChI=1S/C24H21F2NO3/c25-17-5-1-15(2-6-17)22(29)14-13-21-23(16-3-11-20(28)12-4-16)27(24(21)30)19-9-7-18(26)8-10-19/h1-12,21-23,28-29H,13-14H2/t21-,22+,23-/m1/s1
|
|||||
| InChIKey |
OLNTVTPDXPETLC-XPWALMASSA-N
|
|||||
| CAS Number |
CAS 163222-33-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 409.4 | Topological Polar Surface Area | 60.8 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10250673
,103326446
,104179182
,104253290
,104428106
,11528896
,117884262
,12014979
,124658886
,124757411
,124801398
,125164215
,126592989
,126621626
,126653456
,126667079
,127315811
,127315812
,127315813
,127315814
,127315815
,127315816
,127315817
,127315818
,127315819
,127315820
,127315821
,127315822
,127315823
,127315824
,128826430
,131854738
,134338005
,135107180
,14879623
,14928749
,26719841
,46226990
,46386640
,46507625
,49681809
,49693660
,50539938
,53787196
,57347010
,71821424
,7849028
,81146074
,92308446
,92718207
|
|||||
| ChEBI ID |
ChEBI:49040
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| BSEP | Transporter Info | Bile salt export pump | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [2] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [5] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [6] | ||
| References | ||||||
| 1 | Ezetimibe was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Complex pharmacokinetic behavior of ezetimibe depends on abcc2, abcc3, and abcg2. Drug Metab Dispos. 2009 Aug;37(8):1698-702. | |||||
| 3 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | |||||
| 4 | Intestinal expression of P-glycoprotein (ABCB1), multidrug resistance associated protein 2 (ABCC2), and uridine diphosphate-glucuronosyltransferase 1A1 predicts the disposition and modulates the effects of the cholesterol absorption inhibitor ezetimibe in humans. Clin Pharmacol Ther. 2006 Mar;79(3):206-17. | |||||
| 5 | A LC-MS/MS method to quantify the novel cholesterol lowering drug ezetimibe in human serum, urine and feces in healthy subjects genotyped for SLCO1B1. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Jan 2;830(1):143-50. | |||||
| 6 | Pharmacokinetic and pharmacodynamic interactions between the immunosuppressant sirolimus and the lipid-lowering drug ezetimibe in healthy volunteers. Clin Pharmacol Ther. 2010 Jun;87(6):663-7. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
