Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00124
|
|||||
| Drug Name |
Fluvastatin
|
|||||
| Synonyms |
(+)-(3R,5S)-fluvastatin; (-)-(3S,5R)-fluvastatin; (3R,5R,6E)-7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3R,5R,6E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3R,5S)-7-[3-(4-fluorophenyl)-1-propan-2-ylindol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-rel-7-[3-(4-Fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid; (3S,5R,6E)-7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3S,5R,6E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (6E)-7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (6E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (E)-7-[3-(4-fluorophenyl)-1-propan-2-ylindol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (E,3R,5S)-7-[3-(4-fluorophenyl)-1-propan-2-ylindol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (E,3S,5R)-7-[3-(4-fluorophenyl)-1-propan-2-ylindol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (Z,3R,5S)-7-[3-(4-fluorophenyl)-1-propan-2-ylindol-2-yl]-3,5-dihydroxyhept-6-enoic acid; 7-(3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl)-3,5-dihydroxy-6-heptenoate; 7-[3-(4-fluorophenyl)-1-propan-2-ylindol-2-yl]-3,5-dihydroxyhept-6-enoic acid; Canef; Canef(TN); Cranoc; Fluindostatin; Fluvas; Fluvas (TN); Fluvastatin & Primycin; Fluvastatin (INN); Fluvastatin [INN:BAN]; Fluvastatina; Fluvastatina [INN-Spanish]; Fluvastatine; Fluvastatine [INN-French]; Fluvastatinum; Fluvastatinum [INN-Latin]; Lescol; Lescol (TN); Lescol XL; Vastin (TN); XU 62320; XU-62320
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hypercholesterolemia [ICD11:5C80.0] | Approved | [1] | |||
| Therapeutic Class |
Anticholesteremic Agents
|
|||||
| Structure |
|
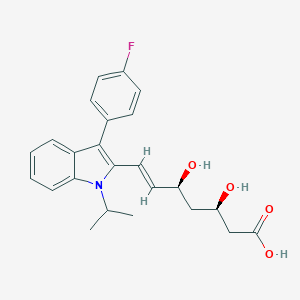 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H26FNO4
|
|||||
| Canonical SMILES |
CC(C)N1C2=CC=CC=C2C(=C1C=CC(CC(CC(=O)O)O)O)C3=CC=C(C=C3)F
|
|||||
| InChI |
InChI=1S/C24H26FNO4/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30)/b12-11+/t18-,19-/m1/s1
|
|||||
| InChIKey |
FJLGEFLZQAZZCD-MCBHFWOFSA-N
|
|||||
| CAS Number |
CAS 93957-54-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 411.5 | Topological Polar Surface Area | 82.7 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
3.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
100024002
,10299838
,103043439
,103293858
,104170157
,104635874
,10584685
,10852031
,117368355
,121362529
,126667384
,130408282
,131378297
,135650279
,136352753
,136910868
,137201916
,143038598
,144075757
,144213082
,14981407
,152164360
,152245639
,162221786
,163620805
,163686130
,165235894
,172080782
,175269733
,175611054
,176253518
,176484235
,24775765
,36888653
,46505668
,47291222
,48413791
,48416032
,49835750
,49857384
,50758963
,53787111
,56312811
,56313633
,57404724
,7979295
,822161
,85856290
,91613309
,92308982
|
|||||
| ChEBI ID |
ChEBI:5136
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [4] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [4] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1B1 | Transporter Info | Km =1.4 microM | Chinese hamster ovary (CHO) cells-OATP1B1 | [4] | |
| OATP1B1 | Transporter Info | Km =3.5 microM | Chinese hamster ovary (CHO) cells-OATP1B1 | [4] | ||
| OATP1B1 | Transporter Info | Km =31.1 microM | Oocytes-OATP1B1 | [6] | ||
| OATP1B3 | Transporter Info | Km =7 microM | Madin-Darby canine kidney cells (MDCKII)-OATP1B3 | [7] | ||
| OATP2B1 | Transporter Info | Km =0.75 microM | Human embryonic kidney cells (HEK293)-OATP2B1 | [4] | ||
| References | ||||||
| 1 | Fluvastatin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Evaluation of the usefulness of breast cancer resistance protein (BCRP) knockout mice and BCRP inhibitor-treated monkeys to estimate the clinical impact of BCRP modulation on the pharmacokinetics of BCRP substrates. Pharm Res. 2015 May;32(5):1634-47. | |||||
| 3 | SLCO1B1 polymorphism and sex affect the pharmacokinetics of pravastatin but not fluvastatin. Clin Pharmacol Ther. 2006 Oct;80(4):356-66. | |||||
| 4 | Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos. 2007 Aug;35(8):1308-14. | |||||
| 5 | A novel screening strategy to identify ABCB1 substrates and inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2009 Jan;379(1):11-26. | |||||
| 6 | The effect of SLCO1B1*15 on the disposition of pravastatin and pitavastatin is substrate dependent: the contribution of transporting activity changes by SLCO1B1*15. Pharmacogenet Genomics. 2008 May;18(5):424-33. | |||||
| 7 | Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Mol Pharmacol. 2005 Oct;68(4):1031-8. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
