Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00116
|
|||||
| Drug Name |
Lamivudine
|
|||||
| Synonyms |
(+/-)-(Cis)-1-[2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (+/-)-3TC; (+/-)-BCH-189; (+/-)-SddC; (-)-(2'R,5'S)-1-[2'-Hydroxymethyl-5'-(1,3-oxathiolanyl)]cytosine; (-)-1-((2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine; (-)-1-[(2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (-)-2'-Deoxy-3'-thiacytidine; (-)-BCH 189; (-)-BCH-189; (-)-NGPB-21; (-)-SddC; (-)-beta-L-2',3'-Dideoxy-3'-thiacytidine; (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one; 2',3' Dideoxy 3' thiacytidine; 2',3'-Dideoxy-3'-thiacytidine; 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (+/-)-(Cis); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Galanthus Nivalis Agglutinin (GNA); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Hippeastrum hybrid agglutinin(HHA); 3'-Thia-2',3'-dideoxycytidine; 3TC; 3TC & GNA; 3TC & SST; 3TC (AIDS INITIATIVE) (AIDS INITIATIVE); 3TC and NV-01; 3TC, Zeffix, Heptovir, Epivir, Epivir-HBV, Lamivudine; 4-Amino-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1H)-pyrimidinone; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one; BCH 189; BCH-189; BCH-790; BCH189; Beta-L-(-)-2',3'-dideoxy-3'-thiacytidine & Sho-Saiko-To; Beta-L-2',3'-Dideoxy-3'-thiacytidine; Beta-L-3'-Thia-2',3'-dideoxycytidine; DRG-0126; DTHC; Epivir; Epivir (TN); Epivir(TM); Epivir-HBV; Epivir-HBV (TN); GG-714; GR-109714X; GR109714X; HHA & 3TC; HHA & Lamivudine; Hepitec; Heptivir; Heptodin; Heptovir; Heptovir (TN); LMV; Lamivir; Lamivudine & GNA; Lamivudine (JAN/USP/INN); Lamivudine [USAN:BAN:INN]; Lamivudine [USAN:INN:BAN]; Lamivudine, (2S-cis)-Isomer; Zeffix; Zeffix (TN); Zefix
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic hepatitis B infection [ICD11:1E51.0] | Approved | [1] | |||
| Human immunodeficiency virus infection [ICD11:1C62.Z] | Approved | [1] | ||||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
| Structure |
|
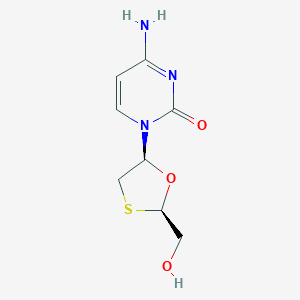 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H11N3O3S
|
|||||
| Canonical SMILES |
C1C(OC(S1)CO)N2C=CC(=NC2=O)N
|
|||||
| InChI |
InChI=1S/C8H11N3O3S/c9-5-1-2-11(8(13)10-5)6-4-15-7(3-12)14-6/h1-2,6-7,12H,3-4H2,(H2,9,10,13)/t6-,7+/m0/s1
|
|||||
| InChIKey |
JTEGQNOMFQHVDC-NKWVEPMBSA-N
|
|||||
| CAS Number |
CAS 134678-17-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 229.26 | Topological Polar Surface Area | 113 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
-0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103195019
,103977094
,104170203
,104253324
,104321740
,11528367
,117588081
,118048664
,12014700
,12016245
,121362453
,124658974
,124757443
,124892101
,125164247
,126584422
,126592947
,126625491
,126656696
,126665392
,127310181
,127310182
,14798125
,15121620
,24277302
,26719826
,29215254
,3727051
,3727058
,43118163
,46386600
,46507855
,49681736
,50140269
,57314135
,596236
,601313
,643736
,7847419
,7979719
,81093205
,811475
,8187080
,85279382
,87560180
,92308311
,92712464
,92729822
,9277
,93166191
|
|||||
| ChEBI ID |
ChEBI:63577
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| 2-Oct | Transporter Info | Organic cation transporter 2 | Substrate | [3] | ||
| BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | 2-Oct | Transporter Info | Km =46.3 microM | Oocytes-OCT2 | [3] | |
| References | ||||||
| 1 | Salicylic acid was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008 Aug;36(8):1616-23. | |||||
| 3 | Genetic variants of organic cation transporter 1 (OCT1) and OCT2 significantly reduce lamivudine uptake. Biopharm Drug Dispos. 2012 Apr;33(3):170-8. | |||||
| 4 | The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine. Br J Clin Pharmacol. 2007 Nov;64(5):645-54. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
