Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00112
|
|||||
| Drug Name |
Irinotecan
|
|||||
| Synonyms |
(+)-Irinotecan; (4S)-4,11-DIETHYL-4-HYDROXY-3,14-DIOXO-3,4,12,14-TETRAHYDRO-1H-PYRANO[3',4':6,7]INDOLIZINO[1,2-B]QUINOLIN-9-YL 1,4'-BIPIPERIDINE-1'-CARBOXYLATE; (4S)-4,11-Diethyl-4-hydroxy-3,14-dioxo-4,12-dihydro-1H-pyrano[3,4-f]quinolino[2,3-a]indolizin-9-yl 4-piperidylpiperidinecarboxylate; Biotecan; Biotecan (TN); CP0; Campto (TN); Camptosar; Camptosar (TN); Camptosar, Campto, CPT-11, Irinotecan; IRINOTECAN HYDROCHLORIDE Trihydrate; IRINOTECAN, CPT-11; Irinotecan (INN); Irinotecan (TOPO1 inhibitor); Irinotecan Hcl; Irinotecan [INN:BAN]; Irinotecan hydrochloride; Irinotecanum; Irinotecanum [INN-Latin]
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Colorectal cancer [ICD11:2B91] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
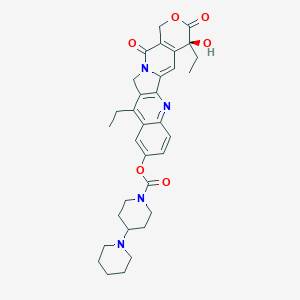 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C33H38N4O6
|
|||||
| Canonical SMILES |
CCC1=C2CN3C(=CC4=C(C3=O)COC(=O)C4(CC)O)C2=NC5=C1C=C(C=C5)OC(=O)N6CCC(CC6)N7CCCCC7
|
|||||
| InChI |
InChI=1S/C33H38N4O6/c1-3-22-23-16-21(43-32(40)36-14-10-20(11-15-36)35-12-6-5-7-13-35)8-9-27(23)34-29-24(22)18-37-28(29)17-26-25(30(37)38)19-42-31(39)33(26,41)4-2/h8-9,16-17,20,41H,3-7,10-15,18-19H2,1-2H3/t33-/m0/s1
|
|||||
| InChIKey |
UWKQSNNFCGGAFS-XIFFEERXSA-N
|
|||||
| CAS Number |
CAS 100286-90-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 586.7 | Topological Polar Surface Area | 113 | ||
| Heavy Atom Count | 43 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103169165
,104020044
,104253165
,104321776
,11409441
,117623718
,118049657
,124757074
,124893595
,125163878
,126630981
,126650121
,126663759
,129430288
,134337937
,134338553
,135032920
,136342503
,137001856
,142371089
,143493285
,143493286
,144116083
,14764625
,14911520
,152034388
,152240238
,43118176
,46393294
,46505871
,47811029
,48427687
,49835834
,49894524
,50422254
,51090967
,53788707
,56312891
,56314523
,57288560
,57314141
,6436484
,645391
,7886725
,7979640
,81092817
,8187089
,85789488
,92711320
,96024776
|
|||||
| ChEBI ID |
ChEBI:80630
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [5] | ||
| MRP5 | Transporter Info | Multidrug resistance-associated protein 5 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MRP2 | Transporter Info | Km =48.9 microM | Bile canalicular membrane vesicles-MRP2 | [7] | |
| MRP2 | Transporter Info | Km =90.8 microM | Madin-Darby canine kidney cells (MDCKII)-MRP2 | [4] | ||
| P-GP | Transporter Info | Km =116.1 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [4] | ||
| P-GP | Transporter Info | Km =45.5 microM | Madin-Darby canine kidney cells (MDCKII)-MDR1 | [4] | ||
| References | ||||||
| 1 | Irinotecan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother Pharmacol. 2006 Sep;58(3):374-83. | |||||
| 3 | ATP-Dependent efflux of CPT-11 and SN-38 by the multidrug resistance protein (MRP) and its inhibition by PAK-104P. Mol Pharmacol. 1999 May;55(5):921-8. | |||||
| 4 | Intestinal transport of irinotecan in Caco-2 cells and MDCK II cells overexpressing efflux transporters Pgp, cMOAT, and MRP1. Drug Metab Dispos. 2002 Jul;30(7):763-70. | |||||
| 5 | P-glycoprotein, but not multidrug resistance protein 4, plays a role in the systemic clearance of irinotecan and SN-38 in mice. Drug Metab Lett. 2010 Dec;4(4):195-201. | |||||
| 6 | Celecoxib upregulates multidrug resistance proteins in colon cancer: lack of synergy with standard chemotherapy. Curr Cancer Drug Targets. 2008 Aug;8(5):414-20. | |||||
| 7 | Biliary excretion mechanism of CPT-11 and its metabolites in humans: involvement of primary active transporters. Cancer Res. 1998 Nov 15;58(22):5137-43. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
