Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00108
|
|||||
| Drug Name |
Testosterone
|
|||||
| Synonyms |
(17beta)-17-Hydroxyandrost-4-en-3-one; (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one; 17-Hydroxy-(17-beta)-androst-4-en-3-one; 17-Hydroxy-(17beta)-androst-4-en-3-one; 17-Hydroxy-4-androsten-3-one; 17-beta-Hydroxy-delta(sup 4)-androsten-3-one; 17-beta-Hydroxyandrost-4-en-3-one; 17b-hydroxy-4-androsten-3-one; 17beta-Hydroxy-3-oxo-4-androstene; 17beta-Hydroxy-4-androsten-3-one; 17beta-Hydroxy-delta(sup4)-androsten-3-one; 17beta-Hydroxyandrost-4-en-3-one; 17beta-Hydroxyandrost-4-ene-3-one; 4-Androsten-17-ol-3-one; 4-Androsten-17beta-ol-3-one; 4-androstene-17beta-ol-3-one; 7-beta-Hydroxyandrost-4-en-3-one; AA 2500; Andro 100; AndroGel; Androderm; Androderm (TN); Androgel (TN); Androlin; Andronaq; Andronate 100; Andronate 200; Andropatch; Andropository 200; Androsorb; Androst-4-en-17beta-ol-3-one; Andrusol; Andryl 200; Beta testosterone; CDB 111C; CMC_13449; COL 1621; CP 601B; Cristerona T; Cristerone T; Delta(sup 4)-Androsten-17(beta)-ol-3-one; Delta4-Androsten-17beta-ol-3-one; Delta4-androsten-17b-ol-3-one; Depotest; Everone 200; Geno-cristaux gremy; Halotensin; Homosteron; Homosterone; Intrinsa; LibiGel; Malerone; Malestrone (amps); Malogen in Oil; Malogen, aquaspension injection; Mertestate; Neo-Hombreol F; Neo-testis; Neotestis; Oreton; Oreton F; Oreton-F; Orquisteron; Perandren; Percutacrine androgenique; Primotest; Primoteston; Relibra; Scheinpharm Testone-Cyp; Striant; Striant (TN); Sustanon; Sustanone; Sustason 250; Synandrol F; T-Cypionate; Teslen; Testamone 100; Testandrone; Testaqua; Testiculosterone; Testim; Testim (TN); Testobase; Testoderm; Testoderm Tts; Testogel; Testoject-50; Testolin; Testopel Pellets; Testopropon; Testosteroid; Testosteron; Testosterona; Testosterona [INN-Spanish]; Testosterone (JAN/USP); Testosterone [Androgenic steroids, anabolic]; Testosterone [INN:BAN]; Testosterone and its esters; Testosterone hydrate; Testosterone solution; Testosteronum; Testosteronum [INN-Latin]; Testostosterone; Testoviron; Testoviron Schering; Testoviron T; Testred Cypionate 200; Testrin-P.A; Testro AQ; Testrone; Testryl; Trans-Testosterone; Virilon IM; Virormone; Virosterone
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Osteoporosis [ICD11:FB83.1] | Approved | [1] | |||
| Structure |
|
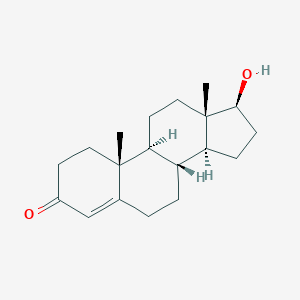 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H28O2
|
|||||
| Canonical SMILES |
CC12CCC3C(C1CCC2O)CCC4=CC(=O)CCC34C
|
|||||
| InChI |
InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1
|
|||||
| InChIKey |
MUMGGOZAMZWBJJ-DYKIIFRCSA-N
|
|||||
| CAS Number |
CAS 58-22-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 288.4 | Topological Polar Surface Area | 37.3 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
3.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10318947
,12012605
,14849069
,14873306
,17389513
,22400338
,24702263
,24870573
,24899970
,24900279
,26717586
,26717589
,26717592
,26717594
,29225027
,3136424
,3817
,46501387
,46505691
,48421877
,48425662
,49703436
,50049744
,50085980
,53789940
,56311126
,56311276
,56312112
,56312748
,56313357
,56313477
,56313560
,56313804
,56314183
,56314208
,56462804
,57288851
,57323115
,584560
,625875
,7847143
,7890744
,8144176
,8153742
,819215
,822362
,822363
,822524
,823294
,841439
|
|||||
| ChEBI ID |
ChEBI:17347
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| References | ||||||
| 1 | Testosterone was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Association of the ABCG2 C421A polymorphism with prostate cancer risk and survival. BJU Int. 2008 Dec;102(11):1694-9. | |||||
| 3 | Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008 Jun 1;14(11):3312-8. | |||||
| 4 | A novel screening strategy to identify ABCB1 substrates and inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2009 Jan;379(1):11-26. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
