Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00094
|
|||||
| Drug Name |
Glibenclamide
|
|||||
| Synonyms |
1-((p-(2-(5-Chloro-o-anisamido)ethyl)phenyl)sulfonyl)-3-cyclohexylurea; 1-(p-(2-(5-Chloro-2-methoxybenzamido)ethyl)benzenesulfonyl)-3-cyclohexylurea; 5-Chloro-N-[4-(cyclohexylureidosulfonyl)phenethyl]-2-methoxybenzamide; 5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxybenzamide; Abbenclamide; Adiab; Apo-Glibenclamide; Azuglucon; Bastiverit; Benclamin; Betanase; Betanese 5; Calabren; Cytagon; Daonil; Daonil (TN); Debtan; Dia-basan; Diabeta; Diabeta (TN); Diabiphage; Dibelet; Duraglucon; Euclamin; Euglucan; Euglucon; Euglucon (TN); Euglucon 5; Euglucon N; Euglykon; G 0639; GBN 5; Gen-Glybe; Gewaglucon; Gilemal; Glamide; Glibadone; Gliban; Gliben; Gliben-Puren N; Glibenbeta; Glibenclamid AL; Glibenclamid Basics; Glibenclamid Fabra; Glibenclamid Genericon; Glibenclamid Heumann; Glibenclamid Riker M; Glibenclamid Riker M.; Glibenclamid-Cophar; Glibenclamid-Ratiopharm; Glibenclamida; Glibenclamida [INN-Spanish]; Glibenclamide (JP15/INN); Glibenclamidum; Glibenclamidum [INN-Latin]; Glibenil; Glibens; Glibesyn; Glibet; Glibetic; Glibil; Gliboral; Glicem; Glidiabet; Glimel; Glimide; Glimidstata; Glisulin; Glitisol; Glubate; Gluben; Gluco-Tablimen; Glucobene; Glucohexal; Glucolon; Glucomid; Glucoremed; Glucoven; Glyben; Glybenclamide; Glybenzcyclamide; Glyburide; Glyburide (USP); Glyburide (micronized); Glyburide [USAN]; Glycolande; Glycomin; Glynase; Glynase (TN); HB 419; HB 420; HB-419; HB-420; HB419; HB420; Hemi-Daonil; Hexaglucon; Humedia; Lederglib; Libanil; Lisaglucon; Maninil; Med-Glionil; Melix; Micronase; Micronase (TN); Micronized glyburide; Miglucan; N-(4-(2-(5-Chloro-2-methoxybenzamido)ethyl)phenylsulfonyl)-N'-cyclohexylurea; N-p-[2-(5-Chloro-2-methoxybenzamido)-ethyl]benzene-sulfonyl-N-cyclohexylurea; N-p-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulfonyl-N'-cyclohexylurea; Nadib; Neogluconin; Norglicem 5; Normoglucon; Novo-Glyburide; Orabetic; Pira; Praeciglucon; PresTab; Prodiabet; Renabetic; Semi-Daonil (TN); Semi-Euglucon; Semi-Gliben-Puren N; Semi-daonil; Sugril; Suraben; Tiabet; U 26452; U-26452; UR 606; Yuglucon
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Diabetes [ICD11:5A10-5A14] | Approved | [1] | |||
| Therapeutic Class |
Hypoglycemic Agents
|
|||||
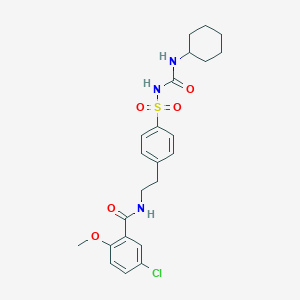
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C23H28ClN3O5S
|
|||||
| Canonical SMILES |
COC1=C(C=C(C=C1)Cl)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3
|
|||||
| InChI |
InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29)
|
|||||
| InChIKey |
ZNNLBTZKUZBEKO-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 10238-21-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 494 | Topological Polar Surface Area | 122 | ||
| Heavy Atom Count | 33 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
4.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321542
,10502519
,11111219
,11112597
,11113353
,11119957
,11120445
,11120933
,11121416
,11121896
,11147040
,11335656
,11360895
,11362485
,11363144
,11365047
,11365706
,11367609
,11368268
,11370241
,11370242
,11372372
,11373210
,11375035
,11375771
,11376430
,11461867
,11466344
,11467464
,11485567
,11486267
,11489632
,11491142
,11493013
,11494064
,14835352
,17405062
,22391414
,24277838
,24895097
,26542333
,3146598
,5047958
,7847402
,7979403
,8147024
,8150168
,8152216
,855894
,9234
|
|||||
| ChEBI ID |
ChEBI:5441
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [3] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP2B1 | Transporter Info | Km =6.26 microM | Human embryonic kidney cells (HEK293)-OATP2B1 | [4] | |
| References | ||||||
| 1 | Glibenclamide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Glyburide transport across the human placenta. Obstet Gynecol. 2015 Mar;125(3):583-8. | |||||
| 3 | Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta. 2006 Nov-Dec;27(11-12):1096-102. | |||||
| 4 | Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005 Apr;33(4):518-23. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
