Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00087
|
|||||
| Drug Name |
Cladribine
|
|||||
| Synonyms |
(2R,3S,5R)-5-(6-amino-2-chloropurin-9-yl)-2-(hydroxymethyl)oxolan-3-ol; 2 Chlorodeoxyadenosine; 2'-Deoxy-2-chloroadenosine; 2-CdA; 2-Chloro-2'-deoxy-beta-adenosine; 2-Chloro-2'-deoxyadenosine; 2-Chloro-6-amino-9-(2-deoxy-beta-D-erythropentofuranosyl)purine; 2-Chlorodeoxyadenosine; 2-chloro-6-amino-9-(2-deoxy-beta-D-erythro-pentofuranosyl)purine; 2-chloro-deoxyadenosine; Adenosine, 2-chloro-2'-deoxy; CL9; Chlorodeoxyadenosine; Cladarabine; Cladaribine; Cladribina; Cladribine (JAN/USAN/INN); Cladribine [USAN:INN:BAN]; Cladribinum; CldAdo; Leustat; Leustatin; Leustatin (TN); Leustatin, 2-chlorodeoxyadenosine, Cladribine; Litak; Movectro;Mylinax; RWJ 26251; RWJ-26251; RWJ-26251-000
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hairy cell leukemia [ICD11:2A82.2] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
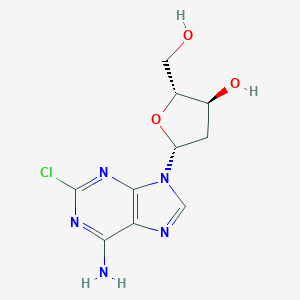 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C10H12ClN5O3
|
|||||
| Canonical SMILES |
C1C(C(OC1N2C=NC3=C(N=C(N=C32)Cl)N)CO)O
|
|||||
| InChI |
InChI=1S/C10H12ClN5O3/c11-10-14-8(12)7-9(15-10)16(3-13-7)6-1-4(18)5(2-17)19-6/h3-6,17-18H,1-2H2,(H2,12,14,15)/t4-,5+,6+/m0/s1
|
|||||
| InChIKey |
PTOAARAWEBMLNO-KVQBGUIXSA-N
|
|||||
| CAS Number |
CAS 4291-63-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 285.69 | Topological Polar Surface Area | 119 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
0.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103602769
,104350170
,118046705
,124659094
,124757075
,124800053
,124886798
,124886799
,125163879
,126624559
,126655830
,128966217
,131314655
,134337856
,134984314
,135683425
,14799875
,14897807
,26719669
,26757803
,29287787
,46386544
,46504588
,48415788
,49865077
,49903916
,49903918
,50104045
,50446026
,56312468
,56312469
,56312470
,56312853
,56422184
,57309572
,57330593
,610461
,71821359
,7848433
,7978577
,7978957
,8165006
,855756
,866156
,87323981
,92308638
,92713835
,99218181
,99431527
,99437023
|
|||||
| ChEBI ID |
CHEBI:567361
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [3] | ||
| ENT3 | Transporter Info | Equilibrative nucleoside transporter 3 | Substrate | [4] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [2] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | ENT3 | Transporter Info | Km =1860 microM | Xenopus Oocytes-ENT3 | [4] | |
| References | ||||||
| 1 | Cladribine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
| 3 | Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008 Sep;7(9):3092-102. | |||||
| 4 | Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005 Apr 22;280(16):15880-7. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
