Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00086
|
|||||
| Drug Name |
L-tryptophan
|
|||||
| Synonyms |
(-)-Tryptophan; (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid; (L)-TRYPTOPHAN; (S)-(-)-2-Amino-3-(3-indolyl)propionic Acid; (S)-(-)-Tryptopha n; (S)-(-)-Tryptophan; (S)-2-Amino-3-(1H-indol-3-yl)propanoic acid; (S)-2-Amino-3-(3-indolyl)propionic acid; (S)-Tryptophan; (S)-a-Amino-1H-indole-3-propanoic acid; (S)-a-Amino-b-indolepropionic acid; (S)-a-Aminoindole-3-propionic acid; (S)-alpha-Amino-1H-indole-3-propanoic acid; (S)-alpha-Amino-beta-indolepropionic acid; (S)-alpha-Aminoindole-3-propionic acid; (S)-alpha-amino-beta-(3-indolyl)-propionic acid; 1-beta-3-Indolylalanine; 151A3008-4CFE-40C9-AC0B-467EF0CB50EA; 1H-Indole-3-alanine; 1H-Indole-3-alanine (VAN); 1beta-3-Indolylalanine; 2-Amino-3-(lH-indol-3-yl)-propanoic acid; 2-Amino-3-indolylpropanoic acid; 2-amino-3-indol-3-ylpropionic acid; 3-Indol-3-ylalanine; Alanine, 3-indol-3-yl; Alpha'-Amino-3-indolepropionic acid; Alpha-Amino-beta-(3-indolyl)-propionic acid; Alpha-amino-beta-(3-indolyl)-pr opionic acid; Alti-Tryptophan; Ardeytropin; EH 121; H-Trp-oh; Indole-3-alanine; Kalma; L-(-)-Tryptophan; L-(-)-Tryptophane; L-TRYPTOPHAN SIGMA GRADE; L-Trp; L-Tryptofan; L-Tryptophan (9CI); L-Tryptophan (JP15); L-Tryptophane; L-Ttp; L-a-Aminoindole-3-propionic acid; L-alpha-Aminoindole-3-propionic acid; L-alpha-amino-3-indolepropionic acid; L-b-3-Indolylalanine; L-beta-3-Indolylalanine; LTR; Lyphan; MT1; Optimax; Pacitron; Propionic acid, 2-amino-3-indol-3-yl; S(-)-1-alpha-Aminoindole-3-propionic acid; Sedanoct; T 0254; TRP NH3+ COOH; TRP-01; Triptofano; Triptofano [Spanish]; Trofan; Trp; Tryptacin; Tryptan; Tryptophan; Tryptophan (H-3); Tryptophan (USP/INN); Tryptophan (VAN); Tryptophan [USAN:INN]; Tryptophan, L-(8CI); Tryptophane; Tryptophane [French]; Tryptophanum; Tryptophanum [Latin]
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Depression [ICD11:6A8Z] | Approved | [1] | |||
| Therapeutic Class |
Antidepressants
|
|||||
| Structure |
|
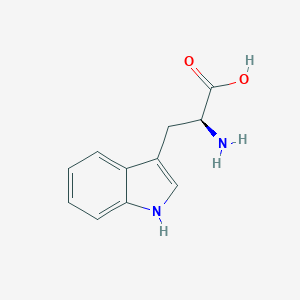 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C11H12N2O2
|
|||||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=CN2)CC(C(=O)O)N
|
|||||
| InChI |
InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1
|
|||||
| InChIKey |
QIVBCDIJIAJPQS-VIFPVBQESA-N
|
|||||
| CAS Number |
CAS 73-22-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 204.22 | Topological Polar Surface Area | 79.1 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-1.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10322924
,10534147
,11109884
,11111851
,11335632
,11360871
,11461843
,11528964
,14710264
,14710293
,14916602
,15195597
,17405739
,24276916
,24277675
,24278135
,24714971
,24770171
,24844224
,24889916
,24900200
,24900575
,25622645
,26703933
,26711994
,26712808
,3134836
,3378
,605298
,6435732
,6436701
,7847088
,7888703
,7890877
,7980851
,8026883
,8144649
,8149576
,8153976
,820596
,821032
,822330
,822480
,823942
,826461
,830210
,834088
,841615
,854680
,855044
|
|||||
| ChEBI ID |
ChEBI:16828
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | LAT2 | Transporter Info | L-type amino acid transporter 2 | Substrate | [2] | |
| MCT10 | Transporter Info | Monocarboxylate transporter 10 | Substrate | [3] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [4] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [5] | ||
| PAT4 | Transporter Info | Proton-coupled amino acid transporter 4 | Substrate | [6] | ||
| References | ||||||
| 1 | Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198. | |||||
| 2 | The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res. 2016 Jan 4;44(D1):D372-9. (ID: 2.A.3.8.20) | |||||
| 3 | Phosphatidylserine synthesis required for the maximal tryptophan transport activity in Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2000 Jan;64(1):167-72. | |||||
| 4 | Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol. 2005 Nov;289(5):C1075-84. | |||||
| 5 | Pharmacogenetics of antidepressants. Front Pharmacol. 2011 Feb 16;2:6. | |||||
| 6 | SLC36A4 (hPAT4) is a high affinity amino acid transporter when expressed in Xenopus laevis oocytes. J Biol Chem. 2011 Jan 28;286(4):2455-60. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
