Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00078
|
|||||
| Drug Name |
Valacyclovir
|
|||||
| Synonyms |
2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl L-valinate; 2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate; 2-{[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methyl]oxy}ethyl L-valinate; Acyclovir-valine; BW-256U; BW256U87; L-Valine ester with 9-((2-hydroxyethoxy)methyl)guanine; L-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl ester; L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9 H-purin-9-yl)methoxy]ethyl ester, monohydrochloride; TBB067866; Talavir; VACV; ValACV; Valaciclovir (INN); Valaciclovir Hcl; Valaciclovir [INN:BAN]; Valaciclovir, Valtrex; Valacyclover Hydrochloric; Valacyclover Hydrochloride; Valacyclovir; Valacyclovir, (L)-isomer; Valtrex; Valtrex (TN); Virval; Zelitrex; Zelitrex (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Herpes simplex virus infection [ICD11:1F00] | Approved | [1] | |||
| Shingles [ICD11:1.00E+91] | Approved | [1] | ||||
| Therapeutic Class |
Antiviral Agents
|
|||||
| Structure |
|
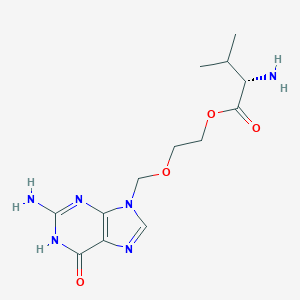 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C13H20N6O4
|
|||||
| Canonical SMILES |
CC(C)C(C(=O)OCCOCN1C=NC2=C1N=C(NC2=O)N)N
|
|||||
| InChI |
InChI=1S/C13H20N6O4/c1-7(2)8(14)12(21)23-4-3-22-6-19-5-16-9-10(19)17-13(15)18-11(9)20/h5,7-8H,3-4,6,14H2,1-2H3,(H3,15,17,18,20)/t8-/m0/s1
|
|||||
| InChIKey |
HDOVUKNUBWVHOX-QMMMGPOBSA-N
|
|||||
| CAS Number |
CAS 124832-27-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 324.34 | Topological Polar Surface Area | 147 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103440109
,104048282
,104178841
,104321602
,117583422
,118313436
,12014356
,124800881
,126631789
,126658129
,126682447
,128045545
,131905333
,134213320
,134221757
,134337933
,135030189
,135659820
,137005923
,137005924
,142590832
,14718342
,14777186
,14801572
,14850688
,14850689
,14874889
,15940044
,160963922
,162782405
,163231563
,163621098
,29215498
,43118121
,46508197
,47589092
,48416691
,50062212
,56422581
,57314102
,626316
,78076275
,7980871
,85279714
,85789653
,89736139
,92309076
,92710527
,96025347
,99775325
|
|||||
| ChEBI ID |
CHEBI:35854
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | ASCT2 | Transporter Info | Alanine/serine/cysteine/threonine transporter 2 | Substrate | [2] | |
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [3] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [4] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OAT3 | Transporter Info | Km =57.9 microM | Proximal tubule (S2) cells-OAT3 | [3] | |
| PEPT1 | Transporter Info | Km =1640 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [5] | ||
| PEPT1 | Transporter Info | Km =2200 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km =2700 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km =5400 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km =6600 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km =7400 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km =2.2 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [7] | ||
| PEPT1 | Transporter Info | Km =7.4 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [7] | ||
| PEPT1 | Transporter Info | Km =292 microM | Human enterocyte-like 2 cells (Caco-2)-PEPT1 | [8] | ||
| PEPT1 | Transporter Info | Km =3.8 microM | Madin-Darby canine kidney (MDCK) cells-PEPT1 | [7] | ||
| PEPT1 | Transporter Info | Km =5940 microM | Oocytes-PEPT1 | [9] | ||
| PEPT1 | Transporter Info | Km =5.9 microM | Xenopus oocytes-PEPT1 | [7] | ||
| References | ||||||
| 1 | Acyclovir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Transport of amino acid-based prodrugs by the Na+- and Cl(-) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004 Mar;308(3):1138-47. | |||||
| 3 | Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther. 2002 Mar;300(3):918-24. | |||||
| 4 | Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998 May 19;246(2):470-5. | |||||
| 5 | Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J Pharmacol Exp Ther. 1999 Apr;289(1):448-54. | |||||
| 6 | Effect of ionization on the variable uptake of valacyclovir via the human intestinal peptide transporter (hPepT1) in CHO cells. Biopharm Drug Dispos. 2000 Jul;21(5):165-74. | |||||
| 7 | Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice. Drug Metab Dispos. 2013 Mar;41(3):608-14. | |||||
| 8 | 5'-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998 Aug;15(8):1154-9. | |||||
| 9 | Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem Biophys Res Commun. 1998 Sep 18;250(2):246-51. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
