Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00071
|
|||||
| Drug Name |
Valsartan
|
|||||
| Synonyms |
(2S)-3-methyl-2-[pentanoyl-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic acid; (S)-N-valeryl-N-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]-methyl}-valine; (s)-2-(n-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)pentanamido)-3-methylbutanoic acid; Aventis brand of valsartan; CEPA brand of valsartan; CGP 48933; CGP-48933; Diovan; Diovan (TN); Diovan, Valsartan; Esteve brand of valsartan; Kalpress; L-Valine, N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-(9CI); Lacer brand of valsartan; Miten; N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine; N-(p-(o-1H-Tetrazol-5-ylphenyl)benzyl)-N-valeryl-L-valine; N-pentanoyl-N-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-L-valine; N-pentanoyl-N-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-L-valine; N-valeryl-N-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)valine; Nisis; Novartis brand of valsartan; Provas; Sanol brand of valsartan; Schwarz brand of valsartan; Tareg; Vals; Valsarran; Valsartan (JAN/USAN/INN); Valsartan [USAN:INN]; Valtan (TN); Valzaar (TN); Walsartan
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11:BA00] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
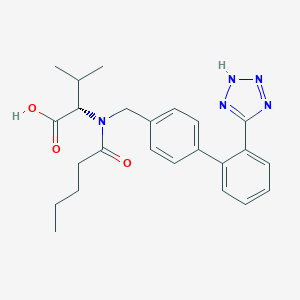
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H29N5O3
|
|||||
| Canonical SMILES |
CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NNN=N3)C(C(C)C)C(=O)O
|
|||||
| InChI |
InChI=1S/C24H29N5O3/c1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23/h6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H,31,32)(H,25,26,27,28)/t22-/m0/s1
|
|||||
| InChIKey |
ACWBQPMHZXGDFX-QFIPXVFZSA-N
|
|||||
| CAS Number |
CAS 137862-53-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 435.5 | Topological Polar Surface Area | 112 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
4.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103292815
,103979540
,104253413
,104321799
,11066615
,11364700
,11367262
,11369824
,11373100
,11374217
,11377988
,11484996
,11488936
,11491646
,11492564
,11495610
,117541337
,117664453
,119526522
,123055291
,124659015
,124757534
,124800101
,125164338
,14856825
,14881173
,26612829
,26719825
,43118184
,46386599
,46509000
,46530915
,47499543
,48393917
,49681716
,49830875
,50062253
,50467452
,53787275
,57314146
,7847466
,81093312
,85788951
,90452226
,92124805
,92308052
,92308462
,92711441
,93166503
,99228303
|
|||||
| ChEBI ID |
ChEBI:9927
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [2] | |
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [3] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [4] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [2] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MRP2 | Transporter Info | Km =30.4 microM | LLC-PK1 cells-MRP2 | [2] | |
| OATP1B1 | Transporter Info | Km =17.8 microM | Chinese hamster ovary (CHO) cells-OATP1B1 | [6] | ||
| OATP1B1 | Transporter Info | Km =1.39 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [2] | ||
| OATP1B3 | Transporter Info | Km =23.5 microM | Chinese hamster ovary (CHO) cells-OATP1B3 | [6] | ||
| OATP1B3 | Transporter Info | Km =18.2 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [2] | ||
| References | ||||||
| 1 | Valsartan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006 Jul;34(7):1247-54. | |||||
| 3 | Prediction of the overall renal tubular secretion and hepatic clearance of anionic drugs and a renal drug-drug interaction involving organic anion transporter 3 in humans by in vitro uptake experiments. Drug Metab Dispos. 2011 Jun;39(6):1031-8. | |||||
| 4 | Regulation of Organic Anion Transporting Polypeptides (OATP) 1B1- and OATP1B3-Mediated Transport: An Updated Review in the Context of OATP-Mediated Drug-Drug Interactions. Int J Mol Sci. 2018 Mar 14;19(3). pii: E855. | |||||
| 5 | High-affinity interaction of sartans with H+/peptide transporters. Drug Metab Dispos. 2009 Jan;37(1):143-9. | |||||
| 6 | Prediction of pharmacokinetic profile of valsartan in human based on in vitro uptake transport data. J Pharmacokinet Pharmacodyn. 2009 Dec;36(6):585-611. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
