Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00046
|
|||||
| Drug Name |
Levofloxacin
|
|||||
| Synonyms |
(-)-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyridol[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate; (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7Hpyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate; (-)-Ofloxacin; (3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid; (R)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (R)-isomer; (S)-(-)-Ofloxacin; (S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (S)-Ofloxacin; Aeroquin; Cravit; Cravit (TN); Cravit Ophthalmic; D-Levofloxacin; DR 3354; DR-3355; DR-3355: L-isomer of ofloxacin; DR3355; Elequine; Floxacin; Floxel; HR 355; HR-355; Iquix; Iquix (TN); L-Ofloxacin; LEVAQUIN IN DEXTROSE 5% IN PLASTIC CONTAINER; LFX; LVX; Leroxacin; Lesacin; Levaquin; Levaquin (TN); Levofloxacin (INN); Levofloxacin [USAN:INN:JAN]; Levofloxacin tablet, suspension or intravenous; Levofloxacine; Levofloxacine [INN-French];Levofloxacino [INN-Spanish]; Levofloxacino; Levofloxacinum; Levofloxacinum [INN-Latin]; Levokacin; Levox; Levoxacin; MP-376; Mosardal; Nofaxin; Ofloxacin; Ofloxacin S-(-)-form; Oftaquix; Oftaquix (TN); Quixin; Quixin (TN); R-Ofloxacin; RWJ 25213-097; RWJ-25213; Reskuin; S-(-)-Ofloxacin; Tavanic; Tavanic (TN); Volequin
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gram-positive & negative bacteria infections [ICD11:1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
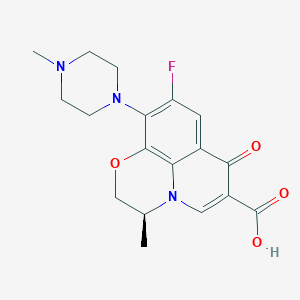 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C18H20FN3O4
|
|||||
| Canonical SMILES |
CC1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O
|
|||||
| InChI |
InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1
|
|||||
| InChIKey |
GSDSWSVVBLHKDQ-JTQLQIEISA-N
|
|||||
| CAS Number |
CAS 100986-85-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 361.4 | Topological Polar Surface Area | 73.3 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
-0.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10250227
,103165325
,104052737
,104179033
,104253435
,11364613
,11367175
,11369737
,11372008
,11374743
,11377899
,11485627
,11489492
,11490810
,11492937
,11495533
,11528725
,12014081
,14876841
,14901428
,24857060
,26612693
,26680408
,26719895
,46225907
,46386771
,46505134
,48185231
,48334772
,49665952
,49681682
,50064059
,50123181
,56314311
,57346857
,57648297
,598046
,76034622
,7979773
,85261747
,85789483
,87558890
,89736102
,92124751
,92307928
,92308354
,92309286
,92710579
,96024810
,9862
|
|||||
| ChEBI ID |
ChEBI:63598
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1A2 | Transporter Info | Km =136 microM | Oocytes-OATP1A2 | [2] | |
| P-GP | Transporter Info | Km =5600 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [4] | ||
| P-GP | Transporter Info | Km =3000 microM | LLC-PK1 cells-MDR1 | [5] | ||
| References | ||||||
| 1 | Levofloxacin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007 Jan-Feb;4(1):85-94. | |||||
| 3 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 4 | Secretory mechanisms of grepafloxacin and levofloxacin in the human intestinal cell line caco-2. J Pharmacol Exp Ther. 2000 Oct;295(1):360-6. | |||||
| 5 | Transport of quinolone antibacterial drugs by human P-glycoprotein expressed in a kidney epithelial cell line, LLC-PK1. J Pharmacol Exp Ther. 1997 Aug;282(2):955-60. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
