Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00035
|
|||||
| Drug Name |
Axitinib
|
|||||
| Synonyms |
AG 013736; AG-013736; AG-013736, Axitinib; AG-13736; AG013736; Axitinib (VEGFR inhibitor); N-methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide; N-methyl-2-[[3-[(E)-2-pyridin-2-ylethenyl]-1H-indazol-6-yl]sulfanyl]benzamide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Advanced renal cell carcinoma [ICD11:2C90] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
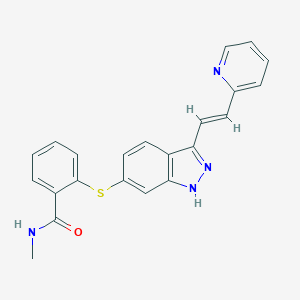
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H18N4OS
|
|||||
| Canonical SMILES |
CNC(=O)C1=CC=CC=C1SC2=CC3=C(C=C2)C(=NN3)C=CC4=CC=CC=N4
|
|||||
| InChI |
InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+
|
|||||
| InChIKey |
RITAVMQDGBJQJZ-FMIVXFBMSA-N
|
|||||
| CAS Number |
CAS 319460-85-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 386.5 | Topological Polar Surface Area | 96 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
4.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
114525306
,118844929
,11972112
,123098392
,124756928
,124950168
,125163735
,126621224
,126647931
,126666999
,131465099
,134339005
,134964394
,135236613
,135697692
,135727420
,136368079
,136920276
,137006067
,137255399
,140075950
,144075334
,144115649
,144207146
,14804960
,152042317
,152258076
,152344032
,160645509
,160646915
,162011700
,162037379
,162112056
,162164950
,162170729
,163395346
,164041895
,164833257
,17397371
,26683791
,43041647
,51067393
,56374259
,56459367
,57370217
,71821495
,85246173
,92718920
,99004612
,99431770
|
|||||
| ChEBI ID |
CHEBI:66910
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Axitinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Meta-analysis of contribution of genetic polymorphisms in drug-metabolizing enzymes or transporters to axitinib pharmacokinetics. Eur J Clin Pharmacol. 2012 May;68(5):645-55. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
