Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00026
|
|||||
| Drug Name |
Fexofenadine
|
|||||
| Synonyms |
2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]piperidin-1-yl}butyl)phenyl]-2-methylpropanoic acid; 2-[4-[1-hydroxy-4-[4-[hydroxy(diphenyl)methyl]piperidin-1-yl]butyl]phenyl]-2-methylpropanoic acid; 4-(1-Hydroxy-4-(4-(hydroxydiphenylmethyl)-1-piperidinyl)butyl)-alpha,alpha-dimethylbenzeneacetic acid; Allegra (TN); Carboxyterfenadine; F 9427; Fastofen (TN); Fexofenadine (INN); Fexofenadine [INN:BAN]; Fexofendine; MDL 16455; Telfast (TN); Terfenadine acid metabolite; Terfenadine carboxylate; Terfenadine-COOH; Terfenidine carboxylate, MDL 16455; Tilfur (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Allergic rhinitis [ICD11:CA08.0] | Approved | [1] | |||
| Therapeutic Class |
Antiallergic Agents
|
|||||
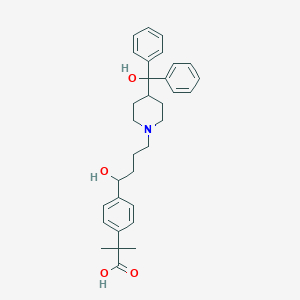
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C32H39NO4
|
|||||
| Canonical SMILES |
CC(C)(C1=CC=C(C=C1)C(CCCN2CCC(CC2)C(C3=CC=CC=C3)(C4=CC=CC=C4)O)O)C(=O)O
|
|||||
| InChI |
InChI=1S/C32H39NO4/c1-31(2,30(35)36)25-17-15-24(16-18-25)29(34)14-9-21-33-22-19-28(20-23-33)32(37,26-10-5-3-6-11-26)27-12-7-4-8-13-27/h3-8,10-13,15-18,28-29,34,37H,9,14,19-23H2,1-2H3,(H,35,36)
|
|||||
| InChIKey |
RWTNPBWLLIMQHL-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 83799-24-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 501.7 | Topological Polar Surface Area | 81 | ||
| Heavy Atom Count | 37 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103238871
,104303248
,11364860
,11367422
,11369984
,11372841
,11373946
,11378153
,11484933
,11488995
,11491736
,11492019
,11495728
,117867878
,121361161
,124749753
,124880162
,124880163
,124891597
,125727652
,126525323
,126683996
,127278637
,127278638
,14835656
,17405051
,29222483
,46504676
,47953966
,48029199
,48029200
,49846688
,49878780
,50105687
,50105688
,5309243
,53777594
,53787836
,57321745
,75782525
,8152130
,85164717
,85209576
,85231056
,85789329
,90341422
,9212
,92304031
,93626300
,96024653
|
|||||
| ChEBI ID |
ChEBI:5050
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | 1-Oct | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [3] | ||
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [4] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [5] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [7] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1A2 | Transporter Info | Km =6.4 microM | Human cervical cancer cell line (Hela)-OATP1A2 | [8] | |
| OATP1B3 | Transporter Info | Km =108 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [5] | ||
| P-GP | Transporter Info | Km =150 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [9] | ||
| References | ||||||
| 1 | Fexofenadine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007 Mar;81(3):362-70. | |||||
| 3 | Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol. 2008 May;73(5):1474-83. | |||||
| 4 | Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol. 2010 Dec 1;80(11):1746-53. | |||||
| 5 | Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005 Oct;33(10):1477-81. | |||||
| 6 | The effects of the SLCO2B1 c.1457C>T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet Genomics. 2011 Feb;21(2):84-93. | |||||
| 7 | Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharmacol Ther. 2005 Aug;78(2):191-201. | |||||
| 8 | OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999 Aug;27(8):866-71. | |||||
| 9 | Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm Res. 2004 Aug;21(8):1398-404. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
