Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00003
|
|||||
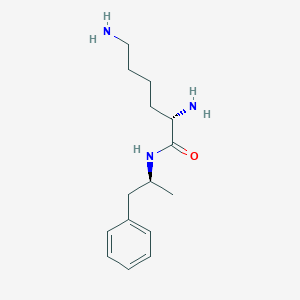
| Drug Name |
Lisdexamfetamine
|
|||||
| Synonyms |
Lisdexamfetamine (INN); NRP104; Vyvanse (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Attention deficit hyperactivity disorder [ICD11:6A05] | Approved | [1] | |||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H25N3O
|
|||||
| Canonical SMILES |
CC(CC1=CC=CC=C1)NC(=O)C(CCCCN)N
|
|||||
| InChI |
InChI=1S/C15H25N3O/c1-12(11-13-7-3-2-4-8-13)18-15(19)14(17)9-5-6-10-16/h2-4,7-8,12,14H,5-6,9-11,16-17H2,1H3,(H,18,19)/t12-,14-/m0/s1
|
|||||
| InChIKey |
VOBHXZCDAVEXEY-JSGCOSHPSA-N
|
|||||
| CAS Number |
CAS 608137-32-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 263.38 | Topological Polar Surface Area | 81.1 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
1.2
|
|||||
| PubChem CID | ||||||
| PubChem SID | ||||||
| ChEBI ID |
ChEBI:135925
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [2] | |
| References | ||||||
| 1 | Lisdexamfetamine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010 Jun 24;6:317-27. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
