Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00209
|
|||||
| Drug Name |
Verteporfin
|
|||||
| Synonyms |
Visudyne (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Age-related macular degeneration [ICD11:9B75.0] | Approved | [1] | |||
| Structure |
|
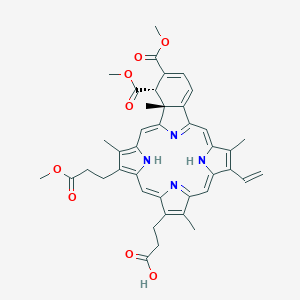 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C41H42N4O8
|
|||||
| Canonical SMILES |
CC1=C(C2=CC3=NC(=CC4=C(C(=C(N4)C=C5C6(C(C(=CC=C6C(=N5)C=C1N2)C(=O)OC)C(=O)OC)C)C)CCC(=O)OC)C(=C3C)CCC(=O)O)C=C
|
|||||
| InChI |
InChI=1S/C41H42N4O8/c1-9-23-20(2)29-17-34-27-13-10-26(39(49)52-7)38(40(50)53-8)41(27,5)35(45-34)19-30-22(4)25(12-15-37(48)51-6)33(44-30)18-32-24(11-14-36(46)47)21(3)28(43-32)16-31(23)42-29/h9-10,13,16-19,38,42,44H,1,11-12,14-15H2,2-8H3,(H,46,47)/t38-,41+/m0/s1
|
|||||
| InChIKey |
YTZALCGQUPRCGW-ZSFNYQMMSA-N
|
|||||
| CAS Number |
CAS 129497-78-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 718.8 | Topological Polar Surface Area | 174 | ||
| Heavy Atom Count | 53 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 10 | |||
| XLogP |
4.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
104222390
,104253378
,124757496
,125164300
,126682291
,135016992
,135692277
,137005637
,137240344
,139249759
,143338983
,14840039
,152159626
,160963806
,174006241
,175269887
,179149780
,198953297
,226397753
,239240257
,249896056
,252158761
,252451139
,33089058
,33089402
,39384497
,46506236
,50064319
,50071237
,53788496
,57362100
,78440468
|
|||||
| ChEBI ID |
CHEBI:60775
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | ABCB6 | Transporter Info | ATP-binding cassette sub-family B member 6 | Substrate | [2] | |
| BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [3] | ||
| References | ||||||
| 1 | Verteporfin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Efficient purification and reconstitution of ATP binding cassette transporter B6 (ABCB6) for functional and structural studies. J Biol Chem. 2013 Aug 2;288(31):22658-69. | |||||
| 3 | The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res. 2007 Apr 15;13(8):2463-70. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Li and Dr. Fu.
